Abstract
To determine the accuracy of patient recall of disease-specific symptoms and quality of life (QoL) in men treated with external beam radiotherapy (EBRT) for localized prostate cancer (LPC). One hundred and five patients enrolled in a prospective study of bladder, bowel, and sexual function, and QoL after radiotherapy for PC were requested to assess their baseline QoL and symptoms before treatment. About one year (mean 14.1 months; range 7–21 months) after treatment, they were asked to recall their baseline QoL and symptoms. Baseline and recall data were compared. Both QoL and symptoms were measured with established instruments, the European Organization for Research and Treatment of Cancer (EORTC) group's QLQ-C30 questionnaire and the Prostate Cancer Symptom Scale (PCSS). Eighty-five of 105 patients (81%) returned the questionnaires. Altogether, the recall of symptoms was poor. Urinary and intestinal symptoms were scored worse at recall than they had actually been at baseline. By contrast, patients tended to remember their baseline sexual function as having been better than it had actually been. The recall of QoL was good. The only QoL domain with a difference between recall and baseline data was that measured in the global health status/QoL scale of the QLQ-C30 questionnaire. The effect did not vary with age or time of follow-up since baseline. Men treated with EBRT for LPC do not accurately recall their pretreatment symptoms or QoL about 1 year after treatment. The accuracy of recall was not affected by time since EBRT (7–21 months). Age did not influence the recall bias. More precise data on impairments in QoL after EBRT in patients with LPC are therefore obtained from baseline and prospective follow-up studies.
Prostate cancer (PC) is the most common cancer disease in Sweden with an incidence of 9 035 new cases in year 2003 Citation[1]. Treatments of localized PC (LPC), such as radical prostatectomy or radiotherapy with modern and improved techniques, will often cure the patient from progressive disease, and prolong survival Citation[2–4]. Each of these approaches is associated with a distinct set of potential risks and benefits. Even if the patient is cured by the treatment, his level of functioning may be decreased and he may sustain treatment-induced complications such as impotence, urinary problems (e.g., incontinence), and problems from the intestinal tract, which may influence both quantity and quality of life (QoL) Citation[5–9]. Whether alterations in the sexual, urinary, and bowel domains are mild or severe, they continue to affect men's lives long after treatment is complete Citation[8], Citation[10–12]. It should be noted, however, that older men with or without LPC do not necessarily always have a good sexual, urinary, and bowel function at baseline Citation[13–16].
Prostate cancer has a long natural history, with 80–90% of patients with small tumors (T0–T2) and well or moderately differentiated tumors living 10–15 years after diagnosis without any treatment besides careful control (watchful waiting) after the diagnosis Citation[17,18]. Evaluation of any treatment-related side effects is therefore of great importance. Consequently, it is of great value to evaluate all aspects of patient outcomes, complications, and survival when taking a treatment decision.
During recent years, there has been a growing interest in more systematic and detailed evaluations of side effects and QoL during and after cancer treatment. Assessments of complications after PC treatment are mostly based on medical records and treating physicians’ estimation of the side effects and many studies recall the patients’ symptoms only retrospectively. When studying the effect of treatment on QoL in men with LPC, it is important to perform baseline measurements before treatment Citation[19]. Prospective assessments can then be used to evaluate the impact of the given treatment on the patients’ QoL. The baseline measure of QoL and symptoms is important because patients with LPC may experience impairments even before treatment Citation[19].
If no baseline pretreatment data are available and a prospective follow-up is impossible to perform, one option is to compare the patients’ QoL and symptoms with those of an age-matched control group Citation[13,20,21]. Another option is to perform a retrospective study with C:\GetARef\Refs\recall.ref #4; recall reporting. However, reports of QoL and symptoms based on recall may not provide valid measures of actual functioning because their accuracy depends on human factors such as perception and memory, which are “inherently subject to distortion” Citation[22].
The self-assessment questionnaire, the Prostate Cancer Symptom Scale (PCSS), covers a wide area of urinary, bowel, and sexual symptoms in patients with LPC. The questionnaire has already been used in retrospective studies Citation[21,23,24] as well as prospective studies Citation[13,20]. Four of these studies included also a comparison with age-matched controls of PC-free men Citation[20,21,23,24].
The aim of the present was to evaluate overall accuracy of recall of QoL and disease-specific symptoms, and establish whether agreement between retrospective recall and actual baseline reports varies as a function of characteristics such as age or time since EBRT.
Material and methods
Patients
Patients were included from a database of 800 patients included in a prospective follow-up study started 1992, examining side effects of EBRT for clinical LPC Citation[13,20,21,24]. Bladder and bowel symptoms, sexual function, and QoL were registered at baseline (i.e., before treatment) and up to 12 years after completed treatment.
All patients in the prospective who had answered the pretreatment questionnaires and have been followed up for at least 6 months and up to 2 years after treatment and were alive at the time for the recall met the criteria for inclusion in the present study. Of these, 105 patients which met the criteria's were selected to obtain the recall assessment.
Recall survey
The same two patient self-assessment questionnaires as used in the baseline registration were sent to the 105 selected patients. The patients were asked to try to think back to the week before their radiotherapy and recall their different symptoms and QoL. The patients’ responses to the recall survey were then compared with their actual response to the baseline survey, the week before EBRT. The study protocol was approved by the Ethical Board at the Northern University Hospital of Umeå, Sweden.
Treatment technique
All patients were treated at the Department of Oncology at Northern University Hospital of Umeå, Sweden. Treatment was given with 20.9 MeV or 50 MeV photons. Radiotherapy was given 5 days a week, with 2 Gy per fraction during 6–8 weeks. The mean total dose was 75.86 Gy. The treatment technique used was 3-D conformal technique.
Instruments
Actual baseline and recall general QoL was assessed with the European Organization for Research and Treatment of Cancer (EORTC) group's QLQ-C30 (version 3) questionnaire.
The original version of the questionnaire (version 1) has been thoroughly validated and cross-culturally tested in cancer patients Citation[25–29]C:\GetARef\Refs\PEK\EXPECTAN\expect.ref #25;. The questionnaire used in this study, the QLQ-C30 (Version 3) questionnaire, is the latest version. The questionnaire contains scales for five domains of functioning: physical, role, emotional, cognitive, and social functioning. It also includes a global health status/QoL scale. Higher mean scores on these scales indicate better functioning and better QoL. Three symptom scales have also been included concerning nausea and vomiting, pain, and fatigue. Six additional, single-symptom items measure the levels of constipation, diarrhea, loss of appetite, sleep disturbance, dyspnea, and the financial impact of the treatment. Higher mean scores on the symptom scales and single-symptom items indicate more symptoms/problems.
Actual baseline disease-specific symptoms were assessed with the validated PCSS questionnaire Citation[30]. This is a self-administered 56-item questionnaire that quantifies prostate-specific symptoms in six domains, namely, urinary function and bother, bowel function and bother, and sexual function and bother. The urinary, bowel, and sexual function scales focus on incontinence, proctitis, and sexual difficulties, respectively, while the bother scales focus on how much the patient is troubled by the dysfunction. The six scales are scored from 0 to 10, with higher scores representing worse function. The PCSS has been shown to be reliable and valid in populations of older men with early and late-stage PC and in older men without PC Citation[13,20,21,23,24,30].
Recalled disease-specific symptoms were assessed with a 48-item short form of the PCSS. To create the recall PCSS formula, we eliminated eight questions containing items from the general scale, concerning issues such as smoking habits and use of medicines. The patients were asked to evaluate their general, urinary, intestinal, and sexual function during the previous week.
Statistical analysis
Mean values were calculated for all domains on both PCSS and QLQ-C30 formulas. Differences between mean values at baseline and recall assessments were evaluated using Wilcoxon's signed-rank test. Internal consistency reliability of the bladder, bowel, and sexual function, and QoL scales was assessed with Cronbach's alpha Citation[31]. Linear regression was used by fitting a linear equation to observed data to calculate the relationship between the degree of the problem and the difference between baseline and recall values. Correlation coefficients were calculated according to Pearson and considered significant if p < 0.05.
Results
Of the 105 questionnaires mailed to the patients, 85 were returned, giving a response rate of 81%. Mean age at baseline was 65.7 years (range 54–77 years). The mean follow-up time since the start of EBRT was 14.1 months (range 7–21 months).
No responders
Those 20 patients’ who did not respond to the recall were younger (61.3 years) than the responders (P = 0.004) at baseline. The no responders reported also more pretreatment bowel symptoms (mean = 2.3) than the responders (mean = 0.86; P = 0.016). No differences could be calculated regarding urinary or sexual symptoms/function.
General function
In the general function domain (e.g., concerning the life situation and limitations in daily life) of the PCSS, patients recalled their baseline function worse than it had actually been. In other words, patients scored their life situation and limitations in daily life due to their PC worse at recall than it had really been at baseline ().
Table I. Differences between actual baseline and recalled symptoms and QOL.
Five out of six domains in the QLQ-C30 questionnaire were remembered well (). The only domain where recall data differed from baseline data was that measured in the global health status/QoL scale, where the patients remembered their function worse than it had actually been (). In the three symptom scales and the six single-symptom items, significant differences between baseline and recall were seen in fatigue (p < 0.001), nausea and vomiting (p = 0.013), dyspnea (p = 0.005), and diarrhea (p = 0.047).
Urinary and bowel symptoms
The precision of recall of patients, of their urinary and bowel symptoms and bother was likewise modest. An overestimation of symptoms at recall was seen when comparing baseline with recall data. Patients remembered their symptoms worse than they had really been at baseline (). The largest difference in any specific item regarding urinary symptoms was “limitations in daily life due to urinary problems” (difference = − 0.87).
The same item regarding bowel symptoms, “limitations in daily life due to intestinal problems”, showed the largest difference in the bowel symptom scale (difference = − 0.62). However, these differences are relatively small because the range of the 10-graded scale is 0–10.
Sexual function
The significance of the difference between baseline and recall data was most conspicuous for sexual function. Patients tended to recall their baseline sexual function as having been better than it had actually been (). This means that about 1 year after completed treatment, patients tended to underestimate their baseline sexual problems. Patients had most difficulty remembering their “desire to have sex” at the recall (difference = 1.68, p < 0.001).
A difference was also seen between baseline and recall outcome regarding sexual activity (p = 0.006). However, the same proportion of patients at baseline (22.0%) as at recall (21.3%) reported that they had not engaged in any sexual activity during the previous year.
Internal consistency
The internal consistency reliability for the items in the PCSS and the QLQ-C30 questionnaire at baseline and recall is given in . Almost all scales exceeded the standard of 0.70 Citation[31]. The internal consistency was higher at recall than at baseline in almost all scales (urinary, intestinal, and sexual function) of the PCSS and the QLQ-C30 QoL questionnaire ().
Table II. Internal Consistency of the PCSS and QLQ-C30 questionnaires.
Age and time to follow-up
No difference was measured between different age groups and quality of recall. However, the elderly patients (>75 years) tended to remember their baseline values worse than younger patients, especially regarding sexual function and sexual bother. A difference of 1.8 between baseline and recall regarding sexual function was seen within the age group 56–65 years and 5.0 within the > 75 years group (ns), respectively.
When stratifying groups of patients according to time since baseline, no difference between the groups could be measured. Remarkably was that those with the shortest follow-up time (<9 months) tended to remember their sexual bother and sexual problems worse than those with longer follow-up time (ns).
Linear regression and correlations
Linear regression showed that both in the sexual function (r2=0.473; p < 0.001; ) and in the sexual bother (r2=0.362; p < 0.001; ) scale, patients with more problems at baseline had greater difficulty remembering their function at recall. This means that those with “no” sexual problem or sexual bother at baseline recalled their situation at baseline with great accuracy about 1 year later. Linear regression showed no difference with regard to the range of urinary or bowel problems.
Figure 1. Linear regression showing the mean difference in sexual function between baseline and recall, and the correlation with mean sexual function at baseline.
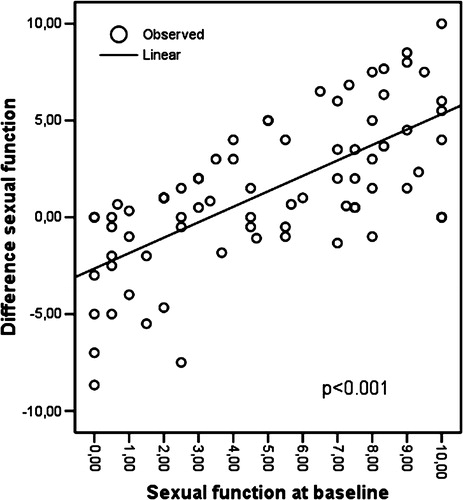
Figure 2. Linear regression showing the mean difference in sexual bother between baseline and recall, and the correlation with mean sexual bother at baseline.
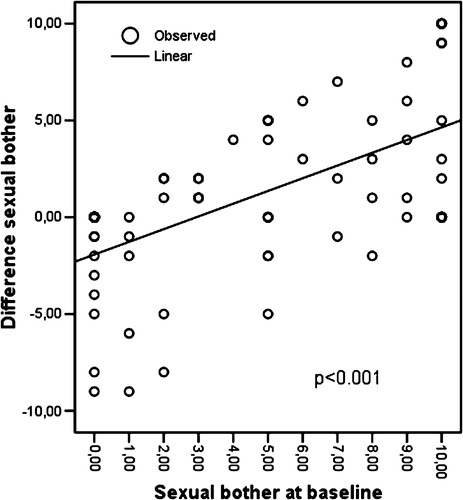
The correlations between baseline and recall were fairly low, especially in the sexual problem domain of the PCSS (). However, all correlations were significant.
Discussion
The present study shows that the accuracy of recall of pretreatment QoL and disease-specific symptoms in men treated with EBRT for LPC is generally low when compared with measurements collected just before treatment.
The best method of studying QoL and disease-specific symptoms of EBRT in men with LPC is to perform prospective assessment with actual baseline values, from before treatment. If no baseline collection of data has been performed and if healthy control groups are unavailable, the method is retrospective assessment. However, there seems to be no difference in precision of recall, whether self-assessment takes place at an earlier time after treatment or whether it takes place later during the follow-up period. Huisman et al. Citation[32] C:\GetARef\Refs\recall.ref #53; have shown that, with increasing follow-up time since baseline, the individuality of the patient has greater impact on the accuracy of the recall than the actual health.
The present study findings are similar to those of Litwin and McGuigan Citation[19], who examined the effect of recall among patients treated with surgery for early-stage PC. Their analysis showed that patients’ overall recall was poor. The patients in their study remembered their baseline health-related QoL as better than it had actually been, especially regarding both urinary and sexual function. By contrast, the present study found an overestimation of symptoms at recall in both urinary and bowel functions. With regard to sexual function, however, an underestimation of sexual problems at recall in comparison with the actual problems at baseline was found. This unexpected finding regarding the underestimation of sexual problems, but not in the other domains, may be explained by a response shift in patients, meaning that they created new individual references because of the experience of trouble when being treated against cancer Citation[20]. Perhaps chronic impairment of the sexual function causes patients to idealize their baseline sexual function and QoL Citation[33]. However, the response shift phenomena are likely to apply also to the other domains, such as urinary and bowel function, and therefore this may not explain the underestimation of sexual problems.
The most accurate way of evaluating QoL in men treated for LPC is to use patients as their own controls Citation[19]. With baseline data on QoL and symptoms, a prospective evaluation of the impairment of QoL is less biased than a recall of the QoL at a later time after treatment. If baseline data assessment is not possible, recall data on the pretreatment QoL need to be evaluated with caution. Normative data of PC disease-free age-matched controls are needed in oncological studies for more correct evaluation of the effect of cancer treatment on QoL Citation[17].
Conclusion
Men treated with EBRT for LPC do not accurately recall their pretreatment symptoms or QoL about 1 year after treatment. In the present study, inaccurate recall was not affected by time since EBRT (7–21 months). Age did not influence the recall bias. More precise data on impairments in QoL after EBRT in patients with LPC are obtained from baseline and prospective follow-up studies.
This investigation was supported by grants from the Swedish Cancer Society, the Research Foundation of the Department of Oncology, University of Umeå, and CAPIO AB.
References
- Socialstyrelsen. The National board of Health and welfare. centre for Epidemiology, Cancer Incidence in Sweden 2003. Statistics Health and Diseases. 2004;10: 10(2004).
- Kupelian P, Kuban D, Thames H, Levy L, Horwitz E, Martinez A, et al. Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: The combined experience of nine institutions in patients treated in 1994 and 1995. Int J Radiat Oncol Biol Phys 2005; 61: 415–19
- Joseph J, Al-Qaisieh B, Ash D, Bottomley D, Carey B. Prostate-specific antigen relapse-free survival in patients with localized prostate cancer treated by brachytherapy. BJU Int 2004; 94: 1235–38
- Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Häggman M, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med 2002; 347: 781–89
- Hunter KF, Moore KN, Cody DJ, Glazener CM. Conservative management for postprostatectomy urinary incontinence. The Cochrane Database of Systematic Reviews 2004; 2: CD001843
- Jani AB, Hellman S. Early prostate cancer: clinical decision-making. Lancet 2003; 361: 1045–53
- Hunskaar S, Burgio K, Diokno A, Herzog A, Hjalmas K, Lapitan MC. Epidemiology and natural history of urinary incontinence. Incontinence: 2nd International Consultation on Incontinence2nd ed, P Abrams, L Cardozo, S Khoury, A Wein. Health Publications, Plymouth UK 2002; 165–200
- Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlén BJ, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med 2002; 347: 790–96
- Helgason AR, Fredrikson M, Adolfsson J, Steineck G. Decreased sexual capacity after external radiation therapy for prostate cancer impairs quality of life. Int J Radiat Oncol Biol Phys 1995; 32: 33–39
- Denton A, Forbes A, Andreyev J, Maher EJ. Non surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. The Cochrane Database of Systematic Reviews 2002; 1: CD003455
- Litwin MS, Sadetsky N, Pasta DJ, Lubeck DP. Bowel function and bother after treatment for early stage prostate cancer: a longitudinal quality of life analysis from CaPSURE. Journal of Urology 2004; 172: 515–19
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J of NCI 2004; 96: 1358–67
- Fransson P, Bergstrom P, Lofroth PO, Widmark A. Prospective evaluation of urinary and intestinal side effects after BeamCath((R)) stereotactic dose-escalated radiotherapy of prostate cancer. Radiother Oncol 2002; 63: 239–48
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61
- Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc 1998; 46: 473–80
- Mulligan T, Moss CR. Sexuality and aging in male veterans: a cross-sectional study of interest, ability, and activity. Arch Sex Behav 1991; 20: 17–25
- Johansson JE, Holmberg L, Johansson S, Bergstrom R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA ;277:467–471. [see comments; published erratum appears in JAMA 1997; 278: 206]
- Johansson JE, Adami HO, Andersson SO, Bergström R, Krusemo UB, Kraaz W. Natural history of localised prostatic cancer. A population-based study in 223 untreated patients. Lancet 1989; 1: 799–803
- Litwin MS, McGuigan KA. Accuracy of recall in health-related quality-of-life assessment among men treated for prostate cancer. J Clin Oncol 1999; 17: 2882–88
- Fransson P, Widmark A. Late side effects unchanged 4-8 years after radiotherapy for prostate carcinoma: a comparison with age-matched controls. Cancer 1999; 85: 678–688
- Fransson P, Widmark A. Self-assessed sexual function after pelvic irradiation for prostate carcinoma. Comparison with an age-matched control group. Cancer 1996; 78: 1066–78
- Herrmann D. Reporting current, past, and changed health status. What we know about distortion. Med Care 1995; 33: 89–94
- Fransson P, Damber JE, Tomic R, Modig H, Nyberg G, Widmark A. Quality of life and symptoms in a randomized trial of radiotherapy versus deferred treatment of localized prostate carcinoma. Cancer 2001; 92: 3111–19
- Widmark A. Self-assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer 1994; 74: 2520–32
- Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, et al. The EORTC core quality of life questionnaire (QLQ-C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer 1995; 31A: 2260–63
- Bjordal K, Kaasa S. Psychometric validation of the EORTC Core Quality of Life Questionnaire, 30-item version and a diagnosis-specific module for head and neck cancer patients. Acta Oncol 1992; 31: 311–21
- Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol 1995; 13: 1249–54
- Osoba D, Zee B, Pater J, Warr D, Kaizer L, Latreille J. Psychometric properties and responsiveness of the EORTC quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual Life Res 1994; 3: 353–64
- King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 1996; 5: 555–67
- Fransson P, Tavelin B, Widmark A. Reliability and responsiveness of a prostate cancer questionnaire for radiotherapy-induced side effects. Support Care Cancer 2001; 9: 187–98
- Cronbach LJ. Coeffecient alpha and the internal structure of tests. Psychometrika 1951; 16: 297–334
- Huisman S, van Dam FS, Aaronson N, Hanewald G. On measuring complaints of cancer patients: some remarks on the time span of the questions. The Quality of Life of Cancer Patients, N Aaronson, J Beckmann. Raven Press, New York 1997; 101–09
- Breetvelt IS, van Dam FS. Underreporting by cancer patients: the case of response-shift. Soc Sci Med 1991; 32: 981–87
