Abstract
The purposes of this study were to evaluate if tumour vascularity by Chalkley counting (TVC) in prostate core biopsies can be a predictor of PSA recurrence after radical prostatectomy in prostate cancer and to estimate the concordance between the TVC in core biopsies and the subsequently examined prostatectomy specimen. All patients, with Gleason score ≤7 in core biopsy, clinical stage T1 or T2 who had a radical prostatectomy during 1990 – 1997 at Sahlgrenska University Hospital, were selected as a primary group. Patients with neoadjuvant hormonal therapy were excluded. The patients were divided into two groups, one with PSA recurrence and one group without PSA recurrence. 25 patients had PSA recurrence during the follow up period and 25 patients from non-recurrence group were randomly selected. TVC was assessed from the prostate tissue by immunostaining against CD34. TVC was statistically significant predictor of PSA relapse. The PSA-free survival rate was only 17% in patients within the highest TVC quartile compared to 67% in patients within the lowest TVC quartile.
The recognition of prostate specific antigen (PSA) has led to a substantial increase of prostate cancer (PC) detection rate in early stages. The situation which today's urologist must handle is to evaluate patients with very early PC and to find a way to distinguish the tumours with high invasive potential, which require immediate treatment, from the tumours with very low invasive ability Citation[1,2].
The sources of information to evaluate the tumours’ aggressiveness can be divided into three categories, namely clinical staging, serum markers and biopsy markers. In serum, usually PSA and free to total PSA ratio are used, in biopsies the number of the cores with cancer, the length of tumour tissue and the Gleason score. The clinical stage is decided based on digital rectal exploration (DRE) and transrectal ultrasound (TRUS) examination of prostate Citation[3].
These prognostic tools are excellent to separate the PC with very poor prognosis from PC with good prognosis, but the major prognostic problem is in the intermediary group. This group consists of patients with T1C or T2 tumours and Gleason score of 3 + 3 or 3 + 4, which are the majority of the newly diagnosed prostate cancer. In this group of patients the ability to predict tumour behaviour is presently limited.
Accurate diagnosis and staging of PC are extremely important for assessment of prognosis and consequently optimizing the therapy selection in this group. In spite of recent advances in this area, the preoperative staging of early PC remains sub-optimal and there is an urgent need of new prognostic markers to increase the accuracy of preoperative PC staging.
Tumour growth is dependent on the formation of new blood vessels, angiogenesis Citation[4,5]. There are currently no markers of the net angiogenic activity of a tumour available to aid investigators in the design of antiangiogenic treatment schemes. The metabolic needs of cancer cells may vary with the tissue of origin and change with tumour progression. TVC may provide a useful independent prognostic indicator of the risk of metastasis and mortality in most carcinomas, melanomas, and some hematologic malignancies Citation[6–8]
Counting or otherwise assessing the occurrence of microvessels in a microscopic field of a tumour tissue section gives an estimate of the net result of phases of angiogenesis and of the angioregression a tumour has went through. TVC basically reflects intercapillary distance and is neither a measure of tumour angiogenic activity, nor a measure of tumour angiogenic dependence Citation[6]. The role of TVC or of a strongly correlating variable such as microvessel density (MVD) as a prognostic marker has been discussed in different tumour types Citation[6–8]. An increased density of capillaries has been demonstrated in PC tissue in radical prostatectomy specimens compared to benign prostate tissue Citation[9,10]. If TVC or MVD could be a clinically useful predictor of prostate cancer recurrence is a matter of discussion. Some investigators find a correlation between MVD/TVC and PSA relapse while others demonstrate the lack of such a correlation Citation[11–13].
In most of the published studies the investigators have used the prostatectomy specimen from radical prostatectomy or TURP material for assessment of TVC/MVD. The prognostic information that is provided by a marker must, however, be available prior to treatment to optimize the therapy selection for the patients. Prior to treatment the urologist has access to core biopsy. One of the questions is if TVC/MVD in the core biopsy can provide similar prognostic information as TVC/MVD in the prostatectomy specimen or TURP material. To our knowledge there have been only few studies in which the biopsy material has been used to assess the TVC/MVD. The reason is probably the small amount of the tumour present in biopsy, which makes the analysis difficult or incomplete. Rogatsch et al. used CD31 antibodies to stain the microvessel in biopsy and prostatectomy specimen. They found a high degree of correlation between MVD in the core biopsies and the prostatectomy specimens when they excluded 22% of cases because of insufficient measurable tumour areas in core biopsy Citation[14].
The purpose of this study was to evaluate whether the TVC in core biopsy could be a clinically useful prognostic marker for PSA recurrence after radical prostatectomy and to investigate the concordance between TVC in core biopsy and prostatectomy specimen.
Patients and methods
Patient selection
Between 1990 – 1997, 363 patients hade undergone radical prostatectomy in Sahlgrenska University Hospital, Gothenburg, Sweden. One hundred and seventy one of 363 patients experience PSA recurrence during the mean follow-up time of 93.7 months (68.6 – 148.7).
Patients who had TURP or cytology as the source of diagnosis were excluded and the number of patients was reduced to 325. Patients with neoadjuvant hormonal therapy were also excluded and 139 patients remained. Ten of 139 had T3 tumor and were excluded. Of remaining 129 patients, 77 had Gleason score < 8. In this group of 77 patients, 25 had PSA-recurrence during the follow up period. Twenty five of remaining 52 patients without PSA-recurrence were randomly selected for further evaluation. Finally 50 patients were selected (T1 – T2, Gleason score 6 or 7, without neoadjuvant hormonal treatment) and divided into two groups: one with PSA recurrence (two consecutive PSA values above 0.1 ng/ml) and the other without PSA recurrence.
Patients follow up
The patients were followed up by a first visit 3 months after prostatectomy and after that every 6 months during the first two years. PSA was tested at all the follow up visits. If no PSA relapse was observed after two years, the patients were followed by annual PSA-measurements.
All the biopsies and the prostate specimens were examined by one pathologist (C-G P), who had no knowledge about the clinical outcome of the patients. TVC in biopsies were added to the prior database including the preoperative prognostic markers.
Statistical evaluation
The power of each preoperative prognostic marker was analyzed in a univariate analysis. A statistician from our department was consulted for the statistical analysis.
Histopathological examination and assessment of TVC
The biopsies were fixed in 4% buffered formalin and were paraffin embedded. Approximately 4 µm thick sections were stained with hematoxylin-eosin or hematoxylin-van Gieson. Estimated Gleason score Citation[15], percent Gleason grade 4/5 and core cancer length were measured in the biopsies.
Prostatectomy specimens from the same patients, prepared in the same way, were reviewed and also Gleason score, percent Gleason grade 4/5, extra capsular extension, positive margin and seminal vesicle invasion were estimated.
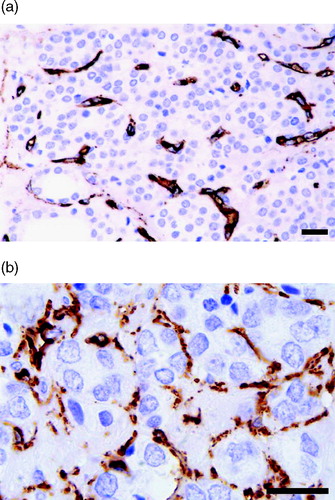
Immunohistochemical staining was made to highlight microvessels. Anti-CD31, anti-CD34 and anti-Factor VIII, were initially used for staining of three randomly selected tumours to clarify which of the antibodies that gave the best result. All stainings were performed with an automatic immunostaining machine; DAKO TechMate™ 500.
Staining with anti-Factor VIII (DAKO, Glostrup, Denmark) revealed relatively few stained microvessels and rather prominent unspecific staining in the stroma. Anti-CD31 (DAKO) also visualized relatively few microvessels but a clean background. Anti-CD34 (DAKO) stained a larger number of microvessels than the other immunostainings, but also often showed some slight unspecific stromal reaction. Based on this comparison, anti-CD34 was chosen for the investigation.
As yet, no ideal endothelial cell (EC) marker, which specifically visualizes all microvessel ECs and only ECs in formalin-fixed and paraffin-embedded tissue, has been developed. In fact, none of the antibodies used here is specific in the strict sense for vessel structures in formalin-fixed and paraffin-embedded tissue, as discussed elsewhere Citation[16]. CD34 is now considered the optimal marker for quantitative tumour vessel assessment because of its robustness and ease of use Citation[8]
Moreover, it is common for investigators to use different criteria, different optical magnification when defining microvessels and different ways to quantify microvessels, as discussed elsewhere Citation[7,8]. This may render comparisons between different studies difficult. In the present study, the general criteria set by Weidner for staining and identifying microvessels in formalin-fixed, paraffin-embedded tumour tissue were followed Citation[17]. Instead of assessing MVD we assessed TVC by Chalkley counting Citation[18]. The reason is that the Chalkley point overlap morphometric technique has abolished one of the highly observer-dependent steps of measuring MVD, namely the frequent decision an observer has to make whether two immunostained and adjacent structures are the reflection of one single or two separate blood vessels. TVC is the objectively assessed relative area that the vessels cover, a measurement that is strongly and significantly associated with vessel number (MVD), i.e. the number of discrete microvessels and with vessel area per unit tissue area Citation[8].
For the anti-CD34 staining different histotechnical variations were used. These include dilutions of the primary antibody, addition of proteolytic digestion with Proteinas K, microwave antigen retrieval with boiling in different buffers like citrate (pH 6.0) and TRIS/EDTA (pH 9.0), and also test to block endogenous biotin activity. The optimal result was achieved when we used DAKO EnVision™ System, which is based on an enzyme-conjugated polymer backbone, which also carries secondary antibody molecules. Antigen retrieval was performed by exposure of the sections to microwave irradiation in TRIS/EDTA buffer (pH 9.0) twice for 7 minutes each time without the use of proteolytic enzyme digestion. The primary antibody was diluted 1:100 and for development DAB was used.
At low power the tumour area was scanned and one ‘hot-spot’ area was identified. Within this ‘hot-spot’ measurements were performed using a Chalkley Point 25 dot eye-piece graticule to quantify TVC. A Leitz Dialux 20 microscope with a 40x objective and 12.5x ocular (500x magnification) was used. The graticule was oriented over the hot-spot region so that as many as possible of the graticule points hit the microvessels as recommended by Fox Citation[19]. In addition to microvessel-like structures that were distinctly stained all single endothelial cells or cell clusters stained in the same way as endothelial cells in the blood vessels were judged as vessels in accordance with Weidner's criteria. Ten measuring areas were examined for each tumour, which corresponds to a total area of 0.75 mm2, an area that is close to the one previously recommended for MVD measurements Citation[17].
In the biopsies, the cancer tissue was often limited, which did not always allow ten view fields to be measured within one and the same hotspot. In these cases, remaining view field were examined within a second, somewhat less richly vascularized hotspot.
Results
Core biopsy
The distribution of the prognostic factors such as PSA, Gleason score, T-stage, total volume of cancer in biopsies and TVC in biopsies in the PSA recurrence and non-recurrence groups are demonstrated in .
Table I. Comparison of preoperative prognostic markers in PSA-recurrence and no-recurrence group, Number of patients*, [Median], (range)
All of the prognostic markers except core cancer length were significantly related to PSA relapse after radical prostatectomy in a univariate analysis ().The PSA and T-stage were the strongest prognostic markers for PSA recurrence (p < 0.0001). Percent of Gleason score 4 in biopsy was a statistically significant prognostic marker (p = 0.0075).
Table II. The result of univariate analysis. The odds ratio for PSA recurrence is calculated for each preoperative prognostic marker.
The overall range of TVC was 2.75 – 13.40. The mean value of TVC in the biopsies was significantly lower in the non-recurrence group compared to the recurrence group (p < 0.02).
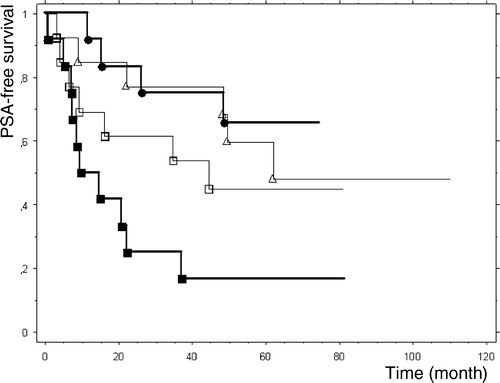
The amount of core cancer length in biopsy was not fully statistically different between the recurrence and non-recurrence groups (p = 0.057). Patients with the lowest TVC quartile had a PSA-free survival of 67% compared to patients with highest TVC quartile who had a PSA-free survival of 17%. The Kaplan-Meier diagram demonstrates the PSA-free survival in different TVC quartiles ().
Figure 1. Kaplan-meier analysis of time to PSA-relapse in relation with different quartiles of TVC in core biopsy. Patients with highest TVC are in first quartile. The PSA-free survival rate were 17% in First quartile▪, 46% in second quartile□, 54% in Third quartile▵ and 67% in Forth quartile• (P = 0.020)
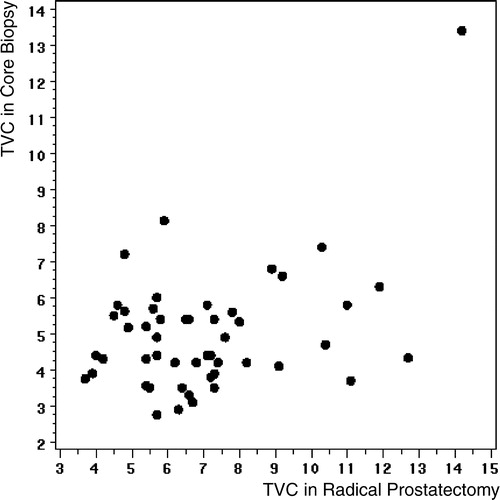
Prostatectomy specimen
In a univariate analysis, TVC calculated from prostatectomy specimen was a predictor of PSA-relapse with an odds ratio of 1.33 (0.99 – 1.79), p = 0.055. The correlation coefficient between TVC in core biopsy and radical prostatectomy specimen was 0.41 (p = 0.0031, ).
Discussion
During a 10-year follow-up period after radical prostatectomy for preoperatively organ confined prostate cancer (PC), 23 – 53% of patients would experience PSA recurrence Citation[20]. The prognosis of PC with Gleason score 8 – 10 is very poor, 33 months actuarial risk of progression is 32% Citation[21]. The major prognostic challenge in PC is in clinically organ-confined tumours with a Gleason score of 6 to 7, due to wide variation of prognosis and the fact that the majority of newly diagnosed PC are in this group. Noguchi et al. demonstrated the difficulty of interpreting the biopsy-based prognostic information in non-palpable tumours. No single parameter in biopsy specimens including PSA, PSA density, and number of positive biopsy, length of tumour in biopsy or per cent Gleason 4/5 was a predictor of tumour aggressiveness Citation[22].
The aim of the patient selection in this study was to find a group of patients with preoperatively comparable prognostic markers but with altered post-operative outcome. By using the data from patients who were operated on between 1990 and 1997 we attain a long follow up time but at the same time we had to exclude a large number of patients due to common use of preoperatively hormonal treatment at that time. There were only 25 patients (T1-2, Gleason < 8) with PSA recurrence and we randomly selected 25 of 52 patients without PSA recurrence for further evaluation.
Several authors have reported the value of MVD/TVC as a prognostic marker in PC Citation[12,14,23,24] but the MVD or TVC in most of the studies was evaluated from prostatectomy specimen or TURP material and not from the core biopsies. In the majority of the patients the prognostic evaluation is needed for therapy selection, in another word the prostatectomy specimen is not available and the prognostic information is based on the core biopsy. The present results of unvariate analysis demonstrate the power of TVC as a prognostic marker in core biopsy.
TVC and MVD, closely correlating variables, have been discussed as a promising prognostic marker of clinical recurrence or PSA recurrence after radical prostatectomy by several authors. Silberman reported that MVD was an independent significant predictor of progression after radical prostatectomy in PC with a Gleason sum of 5 – 7 Citation[13]. In moderately differentiated PC, MVD assessed by Factor VIII-related antigen, was the only statistically significant predictor of clinical recurrence after radical prostatectomy in a univariate analysis and in a Cox’ analysis MVD remained as an independent predictor of biochemical recurrence Citation[23]. Other investigators could not find such a relationship Citation[24,11,25].
Rubin et al. investigated the intraobserver reliability for MVD counting and the conclusion was that MVD counting could be reproduced by a single observer (reliability coefficient 0.82). In the same study MVD assessed by anti-CD31 staining in the radical prostatectomy specimen had no predictive value for PSA failure in men with clinically localized PC Citation[12].
There are few studies of MVD and TVC assessments in core biopsies. In 1996 Bostwik et al. reported that MVD measured by so called optimized microvessel density significantly increased the ability to predict extraprostatic extension of cancer preoperatively when combined with Gleason score and serum PSA concentration Citation[26].
An important quality of a prognostic marker is a consistent concordance between the sample (the biopsy) and the source (the prostatectomy specimen). The measurement of TVC and MVD in core biopsy may be difficult to assess correctly because of the limited amount of tissue present. The imperfect accuracy in the correlation between TVC in the biopsy and the radical prostatectomy specimen prompts prudent interpretation of the results. In fact the same problem exists even with Gleason score evaluation, especially in patients with small amount of tumour in biopsies Citation[27].
Despite the difficulties in the assessment of TVC in core biopsy and intermediary correlation between core biopsy and prostatectomy specimen, the univariate analysis displayed that TVC in core biopsy is a predictor of PSA recurrence after radical prostatectomy in the present study. In the Kaplan-Meier diagram there is a chronological order of the number of patients with PSA recurrence according to MVD quartiles. The patients in the highest TVC quartile had PSA recurrence of 67% compared to 17% in the lowest TVC quartile (). The result of the present explorative study confirms the previous finding of other authors concerning the correlation between MVD/TVC and the aggressiveness of the prostate cancer.
To profoundly investigate the value of TVC/MVD as an independent prognostic marker of PSA recurrence after radical prostatectomy a multivariate analysis including the traditional prognostic markers (PSA, Gleason score, T-stage) is mandatory. There are studies in which authors could recognize MVD as a powerful prognostic marker in an univariate analysis but in the multivariate analysis the MVD was not a significant predictor of recurrence/disease free survival Citation[24,25]. We performed a multivariate analysis and could not find that the TVC was a statistically significant predictor. However due to few patients in this explorative study, the significance of the multivariate analysis result is of limited value.
In future, the optimizing of accuracy of MVD/TVC assessment as well as increasing -the concordance between core biopsy and radical prostatectomy specimen might be achievable by increasing the number of biopsies, which could result in increasing amount of tumor tissue for histopathological diagnosis and quantitative microvessel assessment. One may also have to analyze whether the slower fixation of the cancer tissue in operation specimens as compared with in core biopsies differently affects the expression of endothelial cell epitopes for anti-CD34 or any other immunohistochemical endothelial cell marker.
Conclusion
Tumour vascularity assessment by Chalkley counting (TVC) in core biopsy of PC is a significant prognostic marker of PSA recurrence after radical prostatectomy in univariate analysis in the present study. Future prospective studies including multivariate analysis of the traditional prognostic markers are needed to fully evaluate the role of TVC in preoperative core biopsy specimens as an independent prognostic marker of PSA-relapse after prostatectomy in patients with PC.
Financial support in this project was given by Gothenburg Medical Society, the Swedish Cancer Foundation, M. & B. Gustafson and G. Nilsson foundations.
References
- Dugan JA, Bostwick DG, Myers RP, Qian J, Bergstralh EJ, Oesterling JE. The definition and preoperative prediction of clinically insignificant prostate cancer. Jama 1996; 275: 288–94
- von Eschenbach AC. The biologic dilemma of early carcinoma of the prostate. Cancer 1996; 78: 326–9
- Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. Jama 1997; 277: 1445–51
- Folkman J. The vascularization of tumors. Sci Am 1976; 234: 58–64, 70–3
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71
- Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst 2002; 94: 883–93
- Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 2002; 38: 1564–79
- Fox SB, Harris AL. Histological quantitation of tumour angiogenesis. Apmis 2004; 112: 413–30
- Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignant prostate tissue. Hum Pathol 1993; 24: 220–6
- Eberhard, A, Kahlert, S, Goede, V, Hemmerlein, B, Plate, KH, Augustin, HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. In: Cancer Res; 2000. p., 1388–93.
- Arakawa A, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Prognostic significance of angiogenesis in clinically localized prostate cancer (staining for Factor VIII-related antigen and CD34 Antigen. Prostate Cancer Prostatic Dis 1997; 1: 32–38
- Rubin MA, Buyyounouski M, Bagiella E, Sharir S, Neugut A, Benson M, et al. Microvessel density in prostate cancer: lack of correlation with tumor grade, pathologic stage, and clinical outcome. Urology 1999; 53: 542–7
- Silberman MA, Partin AW, Veltri RW, Epstein JI. Tumor angiogenesis correlates with progression after radical prostatectomy but not with pathologic stage in Gleason sum 5 to 7 adenocarcinoma of the prostate. Cancer 1997; 79: 772–9
- Rogatsch H, Hittmair A, Reissigl A, Mikuz G, Feichtinger H. Microvessel density in core biopsies of prostatic adenocarcinoma: a stage predictor?. J Pathol 1997; 182: 205–10
- Gleason DFatVACURG. Histologic grading and clinicalstaging of prostate carcinoma. in Urologic Pathology. The Prostate 1977:171–198.
- Norrby, K, Ridell, B. Tumour-type-specific capillary endothelial cell stainability in malignant B-cell lymphomas using antibodies against CD31, CD34 and Factor VIII. In: Apmis; 2003. p., 483–9.
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995; 36: 169–80
- Chalkley HW. Method for quantitative morphologic analysis of tissues. J Natl Cancer Inst 1943; 4: 47–53
- Fox SB. Microscopic assessment of angiogenesis in tumors, in Angiogenesis protocols. Humana Press, Totowa, New Jersey 2001
- Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am 1997; 24: 395–406
- Rioux-Leclercq NC, Chan DY, Epstein JI. Prediction of outcome after radical prostatectomy in men with organ-confined Gleason score 8 to 10 adenocarcinoma. Urology 2002; 60: 666–9
- Noguchi, M, Stamey, TA, McNeal, JE, Yemoto, CM. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol 2001;166:104–9; discussion 109–10.
- Halvorsen OJ, Haukaas S, Hoisaeter PA, Akslen LA. Independent prognostic importance of microvessel density in clinically localized prostate cancer. Anticancer Res 2000; 20(5C)3791–9
- Bettencourt MC, Bauer JJ, Sesterhenn IA, Connelly RR, Moul JW. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol 1998; 160: 459–65
- Krupski T, Petroni GR, Frierson HF, Jr, Theodorescu JU. Microvessel density, p53, retinoblastoma, and chromogranin A immunohistochemistry as predictors of disease-specific survival following radical prostatectomy for carcinoma of the prostate. Urology 2000; 55: 743–9
- Bostwick DG, Wheeler TM, Blute M, Barrett DM, MacLennan GT, Sebo TJ, et al. Optimized microvessel density analysis improves prediction of cancer stage from prostate needle biopsies. Urology 1996; 48: 47–57
- Djavan B, Kadesky K, Klopukh B, Marberger M, Roehrborn CG. Gleason scores from prostate biopsies obtained with 18-gauge biopsy needles poorly predict Gleason scores of radical prostatectomy specimens. Eur Urol 1998; 33: 261–70