Background
Several established and widely used oncologic treatment modalities carry the risk of permanent neurotoxicity. Such complications include peripheral neuropathy, encephalopathy, and radiation myelopathy Citation[1,2]. The latter is a typical late reaction with a median latency of 1–2 years Citation[1]. With spinal cord doses below 50 Gy, myelopathy is a very rare complication Citation[1–3]. In recent years, increasing body of evidence suggests that long-term recovery of the irradiated spinal cord allows for retreatment and that certain agents might increase the radiation tolerance in animal models Citation[4,5]. However, few data exist about the influence of pre-existing damage on the tolerance of the spinal cord against radio- and chemotherapy.
Case report
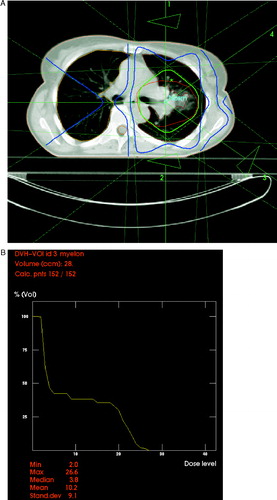
In February 2004, a 45-year old female patient with a family history of Li-Fraumeni syndrome presented with pulmonary metastases from poorly differentiated squamous cell carcinoma of the vagina. The primary tumor (pT1 pN0 M0) had been surgically removed in June 1997. One year later, repeat surgery had been performed because of local recurrence with synchronous inguinal lymph node metastases and soft tissue metastases located in the abdominal wall, followed by adjuvant radiotherapy to these sites. The patient had then been disease-free until February 2002. At that time, pulmonary metastases in the left lung (lingula) were resected. In January 2003, laser-induced thermotherapy was performed for a relapse in the left lung (dorsal lower lobe), followed by two courses of transarterial chemo-embolization in October and November 2003 (mitomycin, gemcitabine, lipiodol). This intervention caused persistent sensory symptoms that developed in the abdomen and both legs (segments below Th 6) in the night after the final drug application. Corticosteroid therapy resulted in slow and partial recovery. However, the patient was fully ambulatory, without any motor deficits and had a Karnofsky performance status of 80%. Magnetic resonance imaging (MRI) showed no structural spinal cord damage. Tibialis sensory evoked potentials on both sides were pathologic. Soon thereafter, further metastases in the left hilus were diagnosed. 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography (FDG-PET) showed no extrathoracic disease manifestation. The hilar lesions were treated with 3-D conformal radiotherapy until March 2004 (30 fractions of 2 Gy, 5 fractions per week, 15 MV photons, 4 coplanar beams). The aim of treatment planning was to reduce the dose to the spinal cord as much as possible. As can be seen from , the median dose to this structure was only 4% (maximum dose 27%, i.e. less than 20 Gy with very low doses per fraction). Radiotherapy was completed without acute grade II or higher skin, mucosal or pulmonal toxicity. However, a slight worsening of the sensory deficits during treatment was noted. A few weeks after radiotherapy, the patient developed a pericardial effusion which was treated by diuretics. In September 2004, a pleural relapse in the left lower lobe as well as a pulmonary metastasis in the right upper lobe were diagnosed by computed tomography and FDG-PET. Within the radiotherapy target volume, a partial remission was seen. The patient received two cycles of carboplatin/gemcitabine without remarkable toxicity. However, the lung lesions continued to increase. Therefore, a single course of paclitaxel was given in November (80 mg/m2). Within 2 days of paclitaxel administration, the sensory deficits deteriorated markedly; however, the patient was still able to walk. While chemotherapy was switched to irinotecan 100 mg/m2 day 1, 8, 15, 22, 36 etc., no recovery of the neurologic deficits was seen as to February 2005. A partial remission of the pulmonary metastases was noted. During the course of observation, all imaging studies excluded other reasons for the neurologic symptoms such as spinal cord compression etc.
Discussion
Transarterial chemo-embolization has mainly been used for the treatment of liver tumors. The risk of central nervous system complications, such as lipiodol embolism, appears to be low Citation[6]. Modern radiotherapy schedules also are rarely associated with permanent spinal cord damage, i.e. radiation myelopathy Citation[2,3]. The risk of chemotherapy-induced spinal cord damage is also low, whereas peripheral neuropathy occurs frequently after administration of certain drugs, including cisplatin and taxanes Citation[7,8]. In a large randomized trial Citation[7], carboplatin plus either docetaxel or paclitaxel led to neurosensory toxicity ≥grade II in 11% vs. 30% of patients (p < 0.001) and to neuromotor toxicity in 3% vs. 7% (p < 0.001). Comparable findings with these two regimens and paclitaxel monotherapy were reported by other authors as well Citation[9,10]. Besides sensory neuropathy, docetaxel was found to induce transient and reversible Lhermitte's sign in 5/87 patients Citation[8]. These data suggest that the compound's toxicity is not always limited to the peripheral nervous system. In the patient with symptoms after transarterial chemo-embolization reported here, a single dose of docetaxel induced marked, and so far irreversible, deterioration of the pre-existing spinal cord damage. Irinotecan and the combination of carboplatin/gemcitabine had no influence on this type of neurotoxicity. We would therefore recommend to consider the increased vulnerability of injured spinal cord when choosing a therapeutic regimen and to inform the patient about the possible consequences of worsening of neurologic sequelae.
References
- Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995; 31: 1093–112
- St Clair WH, Arnold SM, Sloan AE, Regine WF. Spinal cord and peripheral nerve injury: current management and investigations. Semin Radiat Oncol 2003; 13: 322–32
- Falkmer U, Jarhult J, Wersall P, Cavallin-Stahl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncol 2003; 42: 620–33
- Grosu AL, Andratschke N, Nieder C, Molls M. Retreatment of the spinal cord with palliative radiotherapy. Int J Radiat Oncol Biol Phys 2002; 52: 1288–92
- Andratschke NH, Nieder C, Price RE, Rivera B, Tucker SL, Ang KK. Modulation of rodent spinal cord radiation tolerance by administration of platelet-derived growth factor. Int J Radiat Oncol Biol Phys 2004; 60: 1257–63
- Yoo KM, Yoo BG, Kim KS, Lee SU, Han BH. Cerebral lipiodol embolism during transcatheter arterial chemoembolization. Neurology 2004; 63: 181–3
- Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst 2004; 96: 1682–91
- van den Bent MJ, Hilkens PH, Sillevis Smitt PA, van Raaij-van den Aarssen VJ, Bontenbal M, Verweij J. Lhermitte's sign following chemotherapy with docetaxel. Neurology 1998; 50: 563–4
- Hsu Y, Sood AK, Sorosky JI. Docetaxel versus paclitaxel for adjuvant treatment of ovarian cancer: case-control analysis of toxicity. Am J Clin Oncol 2004; 27: 14–8
- Wist EA, Sommer HH, Ostenstad B, Risberg T, Fjaestad K. Weekly one-hour paclitaxel as first-line chemotherapy for metastatic breast cancer. Acta Oncol 2004; 43: 11–4