Abstract
This experience of single agent interferon-α treatment in high-grade osteosarcoma was based on observed anti-osteosarcoma activity in laboratory models and was started before introduction of aggressive combination chemotherapy. From 1971 to 1990, 89 consecutive patients with non-metastatic high-grade osteosarcoma received semi-purified, leukocyte interferon-α as adjuvant treatment. From 1971 to 1984, 70 patients were given a dose of 3 MIU daily for one month followed by 3 times weekly for an additional 17 months. For 19 patients treated from 1985 to 1990 the dose was increased to 3 MIU daily and the treatment duration extended to 3–5 years. All patients underwent surgery prior to interferon treatment. The toxicity was mainly constitutional and long-term toxicity was virtually absent. With a median follow-up of 12 years the observed 10-year metastases-free and sarcoma specific survival rates were 39% and 43%, respectively. Only one of seven survivors after relapse received chemotherapy.
This work suggests activity of interferon-α as adjuvant treatment in high-grade osteosarcoma. The efficacy of interferon in combination with standard therapy should be explored in randomized trials.
Interferons are a group of cytokines with pleiotropic effects including immunostimulation, antiangiogenic activity and direct antitumour activity Citation[1–3]. They have shown high activity against osteosarcoma in vitro and in xenograft models Citation[4–6]. Early preclinical data raised hopes to improve the dismal prognosis of osteosarcoma patients treated with amputation alone Citation[7–9] and the availability of semi-purified interferon-α produced by human leukocytes lead to the initiation of interferon treatment for osteosarcoma at the Karolinska Hospital in 1971. Due to an apparent improvement in outcome the series was extended until 1990 Citation[10–12]. At this time, neoadjuvant chemotherapy was introduced in order to improve the attack on micrometastatic disease and to facilitate limb-salvage surgery Citation[13]. However, subsequent prospective multicentre trials have shown that even the most aggressive combination chemotherapy regimes appear unable to lift the survival above 70–75%, and new treatment options are mandatory for further progress Citation[14].
In this context interferon-α may be a candidate agent in combination with chemotherapy, and the present work represents the final report from this single institution experience on interferon-α treatment of high-grade osteosarcoma, combining two patient series and extending the follow-up from previous preliminary reports Citation[10–12].
Patients and methods
Patients
The Karolinska Hospital is the largest Swedish sarcoma centre serving approximately 30% of the national population. One hundred and two consecutive patients with primary osteosarcoma were admitted to Karolinska Hospital between 1971 and 1990. Nine patients had metastases at diagnosis and four patients had low-grade tumours leaving 89 consecutive patients to be presented in this report. Median age was 17 years (range 5–74), the male: female ratio was 1.6:1 and 92% of the tumours were localized to the extremities with 71% originating in the tibia or femur. Six patients (7%) had pelvic tumours and one tumour originated in a rib. Median maximal tumour extension as determined by the soft tissue mass was 8 cm (range 2–20). Informed consent for participation in the study was obtained from all patients or their parents according to institutional guidelines. The diagnosis was established by open biopsy. Due to the apparent improvement of results all patients entered up to May 1976 (n = 28) were subjected to a detailed review by National Cancer Institute scientists who confirmed the diagnosis of high-grade osteosarcoma Citation[10]. Prior to treatment, all patients had conventional radiographs of the affected bone and a chest x-ray. CT scans of the chest were only done on the suspicion of lung metastases. Diagnostic procedures and follow-up schedules have been reported earlier Citation[10–12].
Treatment
All patients underwent surgery. Before 1976, radiotherapy was given preoperatively to the primary tumour area in 10 patients at doses ranging from 10 to 64 Gy Citation[10]. Thereafter, preoperative radiotherapy was omitted and postoperative radiotherapy reserved for a few patients with intra-lesional surgical margins Citation[12]. No patients received prophylactic lung irradiation.
Limb-salvage procedures were performed in 37% of the patients treated before 1985 and in 50% thereafter. The surgical margins were classified according to Enneking Citation[15] and ten patients (11%) achieved an intralesional margin only.
The interferon used was a semi purified preparation of human leukocyte interferon containing different subtypes of interferon-α, provided by Kari Cantell at the Central Public Health Laboratory and the Finish Red Cross Blood Transfusion Service. The purity and the concentration of the preparation improved by various procedures during the study period Citation[16], Citation[17].
The series is divided into two patient cohorts (). Between 1971 and 1984, 70 patients received three million units daily as a single i.m. injection for one month, followed by three doses weekly for another 17 months. Based on laboratory data suggesting a dose-dependent effect of interferon Citation[5], Citation[6] and clinical observations of tumour regrowth after discontinuation of the drug, both dose and treatment duration were increased from 1985 onwards. Thus 19 patients were treated with three million units daily s.c. for three to five years.
Table I. Characteristics of patients receiving adjuvant interferon-α in Stockholm between 1971 and 1990.
Upon relapse treatment was individualized. Local relapse was resected when possible. Thoracotomies for pulmonary metastases were performed increasingly during the trial period and combined with pulmonary radiotherapy (20 Gy) in some patients. In the 1970's, interferon was discontinued at relapse, and salvage chemotherapy containing either methotrexate or doxorubicin was commenced. In the 1980's, interferon treatment was continued or resumed following resection of the recurrent tumour. Chemotherapy was considered for all patients with unresectable relapses, but was not given in combination with interferon. Unfortunately, the details of treatment at relapse are only available for long-term survivors.
Statistical analysis
Metastases-free survival was calculated from the date of biopsy until the date of distant metastases. Sarcoma-specific survival was calculated from the date of biopsy until death from osteosarcoma or treatment-related causes. Survival was estimated with the Kaplan Meier method and the log-rank test was used for comparisons. Prognostic factor analyses were based on sarcoma specific survival. Variables with p-values of 0.2 or less in univariate analyses were entered into a Cox proportional hazard model. P < 0.05 was considered as statistically significant. Computed statistical analyses applied SPSS for Windows (Release 11.5, SPSS Inc., Chicago, IL, USA).
Results
Toxicity
In the first cohort, fever was reported in 62%, pain at the injection site in 38%, hair-loss in 27%, itching erythema in 17%, coryza-like symptoms in 14% and headache, body stiffness, fatigue and excess perspiration in 10% Citation[18]. Local pain, erythema and body-stiffness were markedly reduced for patients treated with the more purified interferon preparation and in general symptoms tended to decline with time. Interferon did not delay wound healing or new bone formation Citation[12]. Body development was not disturbed by the treatment Citation[19]. Within the first five years of this series two patients committed suicide four and six month into treatment (female, 62 years, male 29 years, both femural amputees). In the second cohort, of eleven patients surviving longer then five years, only two decided to discontinue interferon treatment after three years (due to constitutional symptoms of toxicity), whereas nine continued for five years or more. Detailed data on toxicity for the period after 1979 are not available, but the good treatment compliance indicates no major additional toxicity.
Long term outcome
Median follow-up for survivors was 12 years (range 2–16), and all but two surviving patients were followed for at least ten years (two patients were lost to follow up after 24 and 51 months). Two patients died from unrelated causes (cardiac failure and lung cancer, 13 and 16 years after sarcoma diagnosis) and one died 16 years after diagnosis from an unspecified cause.
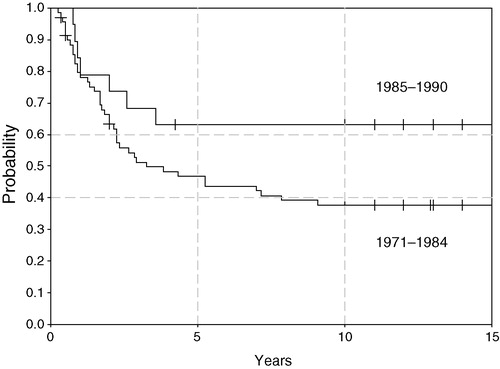
For all 89 patients, metastases-free survival (MFS) and sarcoma specific survival (SSS) at 10 years was 39% (95% confidence interval 29–49%) and 43% (33–54%), respectively (). By treatment period, SSS was 38% in the first cohort and 63% in the second cohort but this difference is not significant (). Median time to metastasis was 8 months (range 1–60); information of the site of metastasis is incomplete but pulmonary metastases were most common.
Figure 1. Sarcoma specific survival (SSS) and metastasis free survival (MFS) in 89 interferon-treated patients.
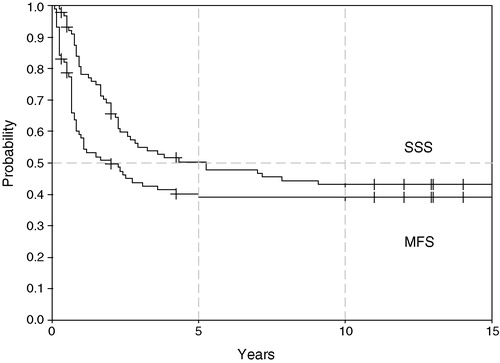
Figure 2. Sarcoma specific survival for two treatment periods, 1971–1984 (n = 70) and 1985–1990 (n = 19) (log rank p = 0.1).
Local relapse was observed in 22% of the patients, and projected local recurrence-free survival at ten years was 75%. The median time to local recurrence was 5.5 months (range 1–60). Patients operated with intralesional margins had a high rate of local recurrence (80%) and a particularly poor outcome with a 10 year SSS of only 10%.
Twenty-eight of the 35 survivors have remained in first remission after systemic treatment with interferon only. Seven of 49 relapsed patients are free of disease after a median of 11.3 years (range 2.7–12.3) from relapse. Two of these had local relapses alone, three had pulmonary metastases and two patients had both local and pulmonary recurrences. All had complete resections of their local relapses and/or metastases, three continued interferon for 5 years and only one survivor from relapse received chemotherapy.
Analysis of prognostic factors
The following variables were included in univariate analyses: age (below versus above median), sex, tumour site (extremity versus other), largest tumour extension (below versus above median), histology (osteoblastic versus non-osteoblastic), margins (intra lesional versus marginal or better), surgery (amputation versus resection) and interferon dose (). Sex, tumour extension, surgical margins and interferon dose were investigated further in multivariate analyses, where female sex and marginal or better surgical margins had independent positive prognostic impact ().
Table II. Univariate analysis of prognostic factors.
Table III. Cox proportional hazards model based on sarcoma specific survival at 10 years of 88 patients (one patient with incomplete data is excluded).
Discussion
In the present series we report a 10-year sarcoma specific survival rate of 43%. With little evidence for a change in the natural history of osteosarcoma yielding a survival of 15–20% with surgery alone Citation[20], Citation[21], and assuming the absence of serious bias, this suggests an effect of interferon-α in primary high-grade osteosarcoma. Although we cannot rule out selective referral of patients with favourable prognosis throughout the series, the number of patients treated matches the expected referral in the Stockholm region, and the age and sex distribution is close to what would be expected. Salvage treatment, and in particular second line chemotherapy in combination with metastasectomy, may save some relapsing patients Citation[22]. However, most survivors in this study are in their first remission after surgery and interferon, and only one long-time survivor received chemotherapy for relapse.
Only one large, randomized trial has explored the effect of interferon in primary osteosarcoma (COSS 80) Citation[23]. For this trial interferon-β was used based on in vitro evidence of superior efficacy compared to interferon-α Citation[4], Citation[23], and was given as maintenance therapy for 22 weeks after completed post-operative chemotherapy. There was no survival difference between the interferon group and the control group. Interferon was however given at a relatively low dose (100 000 IU/kg twice weekly) and for a comparably short time (22 weeks), which may explain the lack of demonstrable effect. Furthermore, interferon was given to patients who already achieved a 60% survival rate, making an effect more difficult to demonstrate.
In our series compliance to treatment was good and all patients continued on interferon according to protocol or until relapse. Two suicides early in the series may be treatment related, and previously published data indicate that depression is associated with interferon therapy Citation[24]. In this context we cannot rule out that patients who have undergone amputation may be particularly vulnerable.
The number of resections with intralesional margins in this experience explains the high rate of local relapse and surgical margins were of independent prognostic impact for survival.
Although a further improvement in survival is suggested in the last cohort of patients, this cannot be attributed to the increase in interferon dose and treatment duration. Not only were the two treatment cohorts separated in time, but tumour size was smaller in the second cohort (). However, interferon trials in other malignancies have shown longer treatment duration and higher weekly dose to result in improved response and survival Citation[25].
Female sex was an independent prognostic factor for improved survival in this series. It is of interest that a subsequent Scandinavian chemotherapy trial also reported gender as an independent risk factor for metastases Citation[13], whereas there is no evidence of a sex difference outside Scandinavia or in Scandinavian patients treated with surgery alone Citation[8], Citation[9], Citation[14], Citation[26]. The underlying mechanism for the sex difference in survival is unclear, but could be due to genetic features in the Scandinavian population affecting response to medical treatment.
During the last two decades, several prospective multicentre studies combining three or four of the most active drugs have been shown to cure at best 70–75% of patients with primary non-metastatic osteosarcoma Citation[13], Citation[26], and further improvement depends on new drugs with novel mechanisms of action. Interferon-α may be a candidate in combination with chemotherapy. Experimental data suggest that multidrug resistant osteosarcoma cell-lines are sensitive to interferon-α Citation[27]. Moreover, interferon has been shown to increase the chemotherapy sensitivity of several drug resistant cell-lines including osteosarcoma lines Citation[27], Citation[28], and has been shown to modulate cytotoxicity by induction of p53 Citation[29]. Interferon's role as maintenance treatment has been extensively studied with favourable results for some cancers Citation[30], Citation[31], and pegylation of interferon-α has reduced toxicity in the treatment of hepatitis and chronic myelogenous leukaemia Citation[32].
In our opinion, interferon-α is an attractive candidate for future studies. An important issue will be how to optimally incorporate interferon with current standard chemotherapy for this disease.
The authors would like to acknowledge: The Norwegian Foundation of Health and Rehabilitation and The Cancer Association of Stockholm for research grants; Kari Cantell, The Central Public Health Laboratory, Helsinki, Finland and The Finnish Red Cross Blood Transfusion Service, Helsinki, Finland for providing the interferon.
References
- Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA 1994; 91: 1198–1205
- Gresser I. Antitumor effects of interferon. Acta Oncol 1989; 28: 347–53
- Strander H. Interferon treatment of human neoplasia. Adv Cancer Res 1986; 46: 1–265
- Einhorn S, Strander H. Is interferon tissue specific?- Effect of human leukocyte and fibroblast interferons on the growth of lymphoblastoid and osteosarcoma cell lines. J Gen Virol 1977; 35: 573–77
- Brosjo O. Osteosarcoma and interferon. Studies of human xenografts in the nude mouse. Acta Orthop Scand Suppl 1989; 229: 1–36
- Hofmann V, Groscurth P, Morant R, Cserhati M, Honegger HP, von Hochstetter A. Effects of leukocyte interferon (E. coli) on human bone sarcoma growth in vitro and in the nude mouse. Eur J Cancer Clin Oncol 1985; 21: 859–63
- Huvos AG. Bone tumors: diagnosis, treatment, and prognosis2nd ed. W.B. Saunders Company, Philadelphia 1991
- Harvei S, Solheim O. The prognosis in osteosarcoma: Norwegian National Data. Cancer 1981; 48: 1719–23
- Lindbom A, Söderberg G, Spjut HJ. Osteosarcoma: a review of 96 cases. Acta Radiol 1961; 56: 1–19
- Adamson A, Aparisi T, Broström LA, Cantell K, Einhorn S, Hall K, et al. Interferon treatment of osteosarcoma. Pontificiae Acad Scient Scripta Varia 1979; 43: 383–406
- Strander H, Bauer HC, Brosjo O, Kreicbergs A, Lindholm J, Nilsonne U, . Adjuvant interferon treatment in human osteosarcoma. Osteosarcoma in adolescents and young adults, G Bennett Humphrey, et al. Kluwer Academic Publishers, Boston 1993; 29–32
- Strander H, Bauer HC, Brosjo O, Fernberg JO, Kreicbergs A, Nilsonne U, et al. Long-term adjuvant interferon treatment of human osteosarcoma. A pilot study. Acta Oncol 1995; 34: 877–80
- Smeland S, Muller C, Alvegard TA, Wiklund T, Wiebe T, Bjork O, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer 2003; 39: 488–94
- Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004; 9: 422–41
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop 1980; 153: 106–20
- Cantell K, Hirvonen S, Kauppinen HL, Myllyla G. Production of interferon in human leukocytes from normal donors with the use of Sendai virus. Methods Enzymol 1981; 78((Pt A))29–38
- Cantell K, Hirvonen S, Koistinen V. Partial purification of human leukocyte interferon on a large scale. Methods Enzymol 1981; 78((Pt A))499–505
- Ingimarsson S, Cantell K, Strander H. Side effects of long-term treatment with human leukocyte interferon. J Infect Dis 1979; 140: 560–3
- Brostrom LA, Adamson U, Filipsson R, Hall K. Longitudinal growth and dental development in osteosarcoma patients. Acta Orthop Scand 1980; 51: 755–9
- Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol 1987; 5: 21–26
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986; 314: 1600–606
- Saeter G, Hoie J, Stenwig AE, Johansson AK, Hannisdal E, Solheim OP. Systemic relapse of patients with osteogenic sarcoma. Prognostic factors for long term survival. Cancer 1995; 75: 1084–93
- Winkler K, Beron G, Kotz R, Salzer-Kuntschik M, Beck J, Beck W, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: results of a Cooperative German/Austrian study. J Clin Oncol 1984; 2: 617–24
- Van Gool AR, Kruit WH, Engels FK, Stoter G, Bannink M, Eggermont AM. Neuropsychiatric side effects of interferon-alfa therapy. Pharm World Sci 2003; 25: 11–20
- Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol 2000; 10: 125–44
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002; 20: 776–90
- Manara MC, Serra M, Benini P, Picci P, Scotlandi K. Effectiveness of type I IFNs in the treatment of multidrug resistant osteosarcoma cells. Int J Oncol 2004; 24: 365–72
- Stein U, Walther W, Shoemaker RH. Modulation of mdr1 expression by cytokines in human colon carcinoma cells: an approach for reversal of multidrug resistance. Br J Cancer 1996; 74: 1384–91
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 2003; 424: 516–23
- Allen IE, Ross SD, Borden SP, Monroe MW, Kupelnick B, Connelly JE, et al. Meta-analysis to assess the efficacy of interferon-alpha in patients with follicular non-Hodgkin's lymphoma. J Immunother 2001; 24: 58–65
- Bjorkstrand B, Svensson H, Goldschmidt H, Ljungman P, Apperley J, Mandelli F, et al. Alpha-interferon maintenance treatment is associated with improved survival after high-dose treatment and autologous stem cell transplantation in patients with multiple myeloma: a retrospective registry study from the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2001; 27: 511–15
- Bukowski RM, Tendler C, Cutler D, Rose E, Laughlin MM, Statkevich P. Treating cancer with PEG Intron: pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation. Cancer 2002; 95: 389–96
