Abstract
In a prospective, controlled, randomised, multicentre study 252 patients with totally resected cutaneous melanoma (248 in stage II–III and 4 in stage IV) were either treated with two doses of dacarbazine (DTIC) followed by a 6-month treatment with 3 MU thrice weekly of highly purified natural interferon-alpha (n = 128; arm A) or received no adjuvant treatment (n = 124; arm B). Treatment was well tolerated. After a median follow-up of 8.5 years ITT analysis showed that the difference in survival was statistically significant with respect to melanoma-related deaths (HR = 0.65, CI = 0.46–0.97, p = 0.022) and close to significance with respect to overall survival (HR 0.71, CI 0.49–1.00, p = 0.052). The risk reduction of melanoma-associated death, calculated by Cox proportional hazards modelling, after adjusting for identified predictive variables, was almost 50% (p = 0.002). The overall efficacy of the treatment appeared to be mainly attributable to effects observed in patients with deep and/or metastasizing tumours (HR 0.60, CI 0.40–0.90, p = 0.013).
The prognosis of patients with cutaneous malignant melanoma, particularly those with deeply growing primary tumours and/or local or regional lymph node metastases (high-risk melanoma), is poor, even if resection of the primary tumour and lymphadenectomy appears successful.
Several adjuvant treatment approaches with recombinant interferon alpha 2 (rIFN-α2) have been tested in clinical studies. Among these, the ECOG study E1684 (rIFN-α2b) showed improvement in both relapse-free survival (RFS) and overall survival (OS) in patients with stage IIb–III tumours when the rIFN was initially administered intravenously in very high doses, up to 20 MU/m2/day Citation[1], Citation[2]. However, upon long-term follow-up, the survival benefit observed was not sustained with time Citation[3], Citation[4]. Likewise, a trend towards a beneficial effect on OS of high-dose rIFN-α2a has been observed Citation[5]. Generally, however, the benefits of high-dose regimens have been modest and this therapy has caused significant toxicity, prompting dose reduction, treatment delays or discontinuation in a significant proportion of patients Citation[1], Citation[2]. Preliminary results obtained after adjuvant treatment with “intermediate” doses of rIFN-α2b (5–10 MU thrice weekly after induction therapy with high-dose IFN-alpha) have not been convincing in terms of OS Citation[6].
Because of the toxicity encountered with high-dose rIFN-α2 regimen in melanoma patients, low-dose regimens (usually 3 MU thrice weekly) have also been attempted as an alternative. Such regimens have generally had limited success Citation[2], Citation[7], Citation[8] although some investigators have reported significant prolongation of relapse-free survival (RFS) in patients in the non-metastasized tumour stages Citation[9–11]. Despite the positive reports on improvement of RFS, a recent systematic meta-analysis of all randomised controlled trials revealed no clear benefit in OS Citation[12].
In contrast to these largely disappointing findings, a preliminary report by Garbe and co-workers Citation[13] suggests that low-dose rIFN-α2a may have a significant effect on OS in patients with cutaneous melanoma and regional lymph node metastases.
In the present study highly purified low-dose natural human interferon alpha (HuIFN-αLe) administered following induction treatment with dacarbazine (DTIC), was used as adjuvant treatment. Because of its antitumour effects in advanced melanoma Citation[14] DTIC was used first which might reduce the number of occult tumour cells or tumour cell clusters prior to HuIFN-αLe therapy. A reason for choosing HuIFN-αLe instead of rIFN-α2 was that HuIFN-αLe had previously demonstrated promising results in small studies for adjuvant therapy Citation[15] and also in studies for combination treatment of advanced melanoma Citation[16]. In addition, a pilot study involving 30 patients and 60 historical controls, suggested that low-dose HuIFN-αLe, administered after two injections of DTIC, may have a beneficial effect on OS and therefore be useful as adjuvant treatment of cutaneous melanoma (Stadler et al., unpublished results).
A further reason to consider HuIFN-αLe rather than rIFN-α2 was that HuIFN-αLe comprises several IFN-α subtypes, some of which are believed to have antiproliferative and immunological effects that are quantitatively or qualitatively different from rIFN-α2 which represents a single IFN-α subtype Citation[17].
Patients and methods
Trial design
This was a randomised prospective clinical study initiated in 1993 to assess clinical efficacy of two cycles of DTIC followed by a 6-month adjuvant treatment with highly purified HuIFN-αLe (Multiferon® ViraNative AB, Umeå, Sweden; arm A) in melanoma patients after complete tumour surgery, in comparison to tumour surgery only (arm B = control group). The trial was conducted at 19 clinical sites in Germany.
The treatment in arm A started with 2 IV injections of DTIC (850 mg/m2 body surface on day 2 of treatment week 1 and 5). For reduction of DTIC-related side effects, antiemetic therapy with ondansetron was recommended. Four weeks after the second DTIC injection, the patients were switched to a 6-months therapy with HuIFN-αLe (3 MU thrice weekly, sc).
At each investigational site the patients were stratified based on melanoma staging as defined by the “Deutsche Dermatologische Gesellschaft” at that time: Stratum 1 (tumour thickness > 1.5 mm), Stratum 2 (intransit and/or satellite metastases) and Stratum 3 (regional lymph node metastases). The randomization list for each site included two blocks (verum vs controls) of ten patients. The random-allocation list was computer-generated by the study data management group for each participating centre prior to study onset (program: “RANDOM” by Dr Wiedey, Constance, Germany).
The patients had to meet the following eligibility criteria before randomisation: Written IRB-approved informed consent; histologically proven melanoma (based on pathological records in each centre and, in cases of stage IIa/b melanoma, after examination by a central pathology unit); surgery with a 2 cm margin, including radical lymphadenectomy and excision of all satellite metastases and/or intransit metastases; randomisation within one month after tumour excision; no previous systemic interferon treatment; age ≥ 18 years; Karnofsky-index ≥ 70; white blood cell count ≥ 2×109 /L; platelet count ≥ 100 x 109 /L; alkaline phosphatase ≤ 300 U/L; AST ≤ 50 U/L; bilirubin ≤ 1.5 mg/dL; cholinesterase ≥ 3 U/L; creatinine ≤ 2.0 mg/dL; hemoglobin ≥ 9 g/dL; no anamnestic coagulopathy; negative pregnancy test; willingness of female patients of child-bearing age to apply contraception during treatment and until three months after scheduled termination of therapy; no known hypersensitivity towards interferon, human serum albumin or imidazolcarboxamide.
The primary objective of the trial was “relapse free survival” (RFS) while the secondary objectives were survival considering melanoma related mortality and treatment tolerability. The trial was reviewed and approved first-line by the ethics committee of the University Medical School of Münster. Additionally, the protocol was approved by the ethics committees of the University Medical Schools of Dresden, Ulm, Frankfurt on the Main, Freiburg, Munich and Magdeburg. The study was performed according to Good Clinical Practice (GCP).
Follow-up
Clinical examination was performed in both study arms after 2 (end of DTIC-therapy), 8 (end of HuIFN-αLe therapy) and 12 months. During the second year of follow-up, patients were examined quarterly, from the third to the fifth year patients were examined at 6-months intervals and from 1999 to 2001 patients were examined once. In both arms all clinical examinations included x-ray of the thorax, echosonography of abdomen and lymph nodes after 8, 12, 18 and 24 months and CT of the thorax, the abdomen and the cranium and bone scanning as optional techniques at any scheduled follow-up. In arm A, blood counts and biochemical analyses were performed before DTIC treatment, then at weeks 2, 4, 6, 8, 9 and 12 and at month 5, 8 and 12 of the follow-up. Patients in arm B had safety laboratory testing at weeks 4 and 8 and after 8 months of their follow-up. Adverse events were recorded in arm A patients at weeks 4, 8, 9 and 12 and at month 5 and 8. The WHO toxicity scale was applied in grading the severity of toxicity.
In order to collect further information on survival and cause of death a longterm followup was conducted during 2003–2004 in all surviving patients including those who had dropped out.
Since this long-term follow-up was implemented two to three years after the completion of the protocol, renewal of consent was required for patients who had been lost or had withdrawn during the initial follow-up. The follow-up was limited to historical collection of patient survival information, and no visit or examination of the patients were required. The patients (or relatives) were informed about the reasons for this long-term follow-up and they were asked to renew their agreement for use of the additional individual data. In no case was the permission refused.
Statistical analyses
For the computation of sample size it was estimated that the mean 5-year relapse rate among patients who undergo surgery only is 45% in stage IIa, 80% in stage IIb and 75% in stage III. A sample size of 236 patients provided a 90% power (α = 0.05) to detect a reduction in the rate of relapse by 20% in arm A compared to the control patients in each of the randomization strata including a calculated drop-out rate of 10%. Since the observed dropout rate during the enrolment period was higher than expected, the final sample size was increased to include 253 patients.
Survival data was analysed by considering melanoma related mortality only (prospectively defined in the protocol) and by the inclusion of all causes of mortality (Overall survival, OS) in exploratory analyses. The subgroup of patients in tumour stage IIb–III (high-risk patients) was additionally analysed in an explorative way.
Relapse and survival outcome was estimated by the Kaplan-Meier method and compared by the use of two-sided log-rank test with a α-value of 0.05. Hazard ratios (HR) with 95% confidence limits (Cl) and p-values were used for the presentation of results. To assess the homogeneity of the distribution of patient/tumour characteristics the χ2 tests and t-tests were used. When the assumption of normal distribution was rejected, non-parametric methods were used (e.g. Kruskal-Wallis test, Mann-Whitney U-test).
The multivariate evaluation of the impact of pre-treatment parameters on survival was performed by Cox proportional hazards modelling, that was found to be appropriate for the assessment of the robustness of the significance levels obtained using log-rank tests. The method was chosen after comparison with several non-proportional methods. Gender, Breslow score, Clark level, histology, age and location of primary tumour were considered as well as first order interactions with treatment. The analysis was performed using the SCORE option in the SAS PHREG procedure. This option selects the subset of predictive variables with the highest score χ2 statistic among all possible model sizes ranging from one explanatory variable to the total number of explanatory variables provided to the analysis procedure. The analysis was performed with the treatment factor forced into the model.
Study drug
HuIFN-αLe, manufactured by Viranative AB (Umeå, Sweden) under the trade mark Multiferon® is produced by a modification of the Cantell method from leucocytes obtained from blood donations. Multiferon® is a highly purified mixture of the natural human interferon alpha subtypes 1, 2, 8, 10, 14 and 21 of human interferon alpha.
Results
Data sets analysed
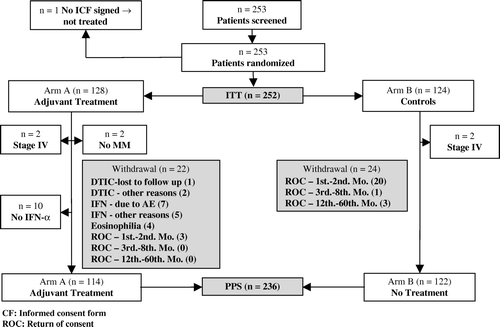
Between March 1993 and June 1997 253 adult patients with malignant melanoma were enrolled in the study within one month after they had undergone surgical resection of their tumour and all detectable local or regional lymph node metastases. One patient did not provide written consent to participate in the study and was not treated. Thus, 252 patients constituted the complete intention-to-treat (ITT) population while two patients with wrong diagnosis (no melanoma histology), four patients with stage IV melanoma and ten patients who never received any injection of HuIFN-αLe were excluded for the explorative analysis of the PerProtocol Selection (PPS) population (n = 236) (). The PPS population consisted of patients who met the protocol inclusion criteria at the time of randomization and who had received at least one injection of Multiferon. Among the patients who never received any injection of HuIFN-αLe four were in stage IIa while six were in stage IIIb. The PPS population was primarily analysed in order to assess the robustness of the results with respect to deviations from the protocol. Explorative analyses were also performed in the “population of high-risk (stage IIb–III) patients. This population consisted of 158 patients, 82 in arm A and 76 in arm B.
In total 46 patients withdrew from the study (22 from arm A and 24 from arm B) (). While in arm A the patients withdrew mostly for safety reasons during follow-up, the patients in arm B withdrew early in the trial shortly after they were informed that they were randomised into the control group.
At the end of the prospective follow-up in 2001, the median follow-up time was 5.5 years and maximum follow-up 7 years. After the long-term follow-up in 2004 the median follow-up time was 8.5 years. At this time 251 of 252 patients had been followed up for 5 years the minimum while the maximum follow-up time was 10 years.
Prognostic factors
The ITT population was well balanced between arms A and B with respect to demographic and tumour-specific factors of potential prognostic relevance (). At the time of enrolment of patients in the study recording of tumour ulceration and numbers of lymph node metastases were not routinely carried out. For these reasons, staging in accordance with the currently used AJCC 2002 classification system was not possible. Testing of centre effects by non-parametric testing (Kruskal-Wallis test) for all tumour stages did not reveal any significant differences.
Table I. Tabulation of demographic and baseline parameters of the ITT population.
Clinical efficacy
Outcome analyses in 2001
The analyses performed in 2001 upon completion of the prospective follow-up revealed that there was no statistically significant difference between arm A patients and arm B patients of the ITT population with respect to RFS or melanoma related death (log rank tests, p = 0.068 and p = 0.97, respectively). Fifty-six (43.8%) patients had relapsed in arm A and 62 (50.0%) patients had relapsed in arm B. The median RFS time for the arm A population was 1 002 days, 541 days longer than in the control group (461 days).
In an exploratory analysis of the patients in tumour stages IIb–III (high-risk population; n = 158; 82 patients in arm A and 76 patients in arm B) the log-rank test revealed a significant difference in RFS between arm A and arm B (p = 0.002) while the difference in melanoma related death was not significant (p = 0.38). The finding of improved RFS in arm A of high-risk patients provided the incentive to conduct an additional follow-up in 2003/2004. A focus was then placed on OS rather than RFS, since the high dropout rate observed in the initial stage of the study would be supposed to confound later calculations of RFS.
Long-term survival follow-up in the ITT population in 2003/2004 (n = 252)
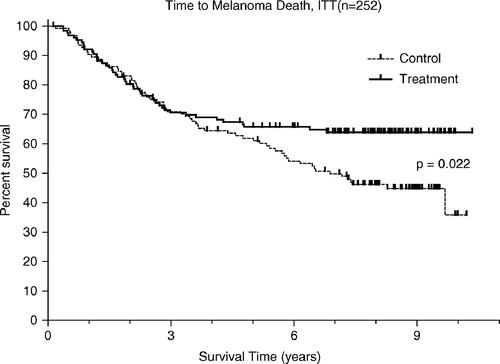
The analysis of the complete ITT population in 2003 and 2004 regarding melanoma-related mortality is shown in . In arm A 45 patients (35.2%) died from melanoma while 67 arm B patients (54.0%) died from this malignancy. The difference between the two groups was statistically significant (HR = 0.65, CI = 0.46–0.97, p = 0.022) in favour of arm A.
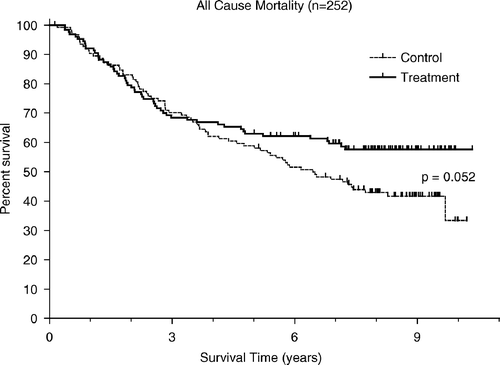
When comparing OS in both groups (considering all causes of mortality), the difference was close to significance (HR = 0.71, CI = 0.49–1.00, p = 0.052). In this population 53 patients (41.4%) died in arm A while 72 patients (58.1%) died in the control group ().
As seen in and three Kaplan Meier curves separate only after the first couple of years following randomisation. Deaths occurring later than five years after randomisation were rare among the arm A patients but common among the controls. In the entire population of arm A patients there were six deaths, which occurred after more than five years; four of these being from causes that apparently were unrelated to melanoma. In contrast, there were 21 deaths in arm B, only one of which was considered unrelated to the patient's melanoma. Thus, in patients who had survived the first five years after randomisation, deaths due to melanoma progression were ten times more common in untreated, than in adjuvant-treated patients.
Long-term OS follow-up in the PPS (n = 236) and high-risk (n = 158) populations (explorative analyses)
In the PPS population, which comprised 114 patients in arm A, 46 (40.4%) died in total. The control group consisted of 122 patients of whom 71 (58.2%) died. The difference between the two groups in terms of OS (based on all cause mortality) was statistically significant (HR = 0.66, CI = 0.46–0.96, p = 0.029) in favour of the arm A patients.
In the high-risk (stage IIb–III) population (n = 158) 39 patients (47.6%) had died in arm A and 57 (75%) in arm B. The difference between the two arms in terms of OS was statistically significant (HR = 0.58, Cl = 0.38–0.86, p = 0.008).
Non-melanoma related death during study
In arm A, a total of eight patients died of non-melanoma related causes (2 caecum cancer, 3 cardio-vascular disease and 3 of unknown cause) while in the control group, a total of five patients died from non-melanoma causes (2 caecum cancer, 1 suicide and 2 of unknown cause). The three patients who died of cardiac disease had stage IIa melanoma at randomization. These patients had a long previous history of cardiovascular disease, and they died years after the completion of the adjuvant treatment.
Predictor variables and hazard ratio in Final Cox Proportional Model
shows the results of the proportional hazards modeling of melanoma-associated mortality. The best subset of predictive variables in the final statistical model is listed with treatment forced into the model. The effect of treatment remains statistically significant at the 0.05 significance level even when five statistically significant explanatory variables were included in the statistical model (p = 0.002). Clark level (I–III vs. IV or V) showed that the hazard was 122% greater for those with higher grades than for lower grades. Histological analysis (SSM, LMM, vs. nodular melanoma, ALM) showed that the hazard was 68% higher for those with SSM or LMM. Analysis of the location of the tumour (extremities, vs. head, neck, trunk) showed that patients with a tumour on the head, neck or trunk had 139% higher hazard than those with tumour on the extremities. There was a 48.5% reduction in the hazard for adjuvant treatment compared to no adjuvant treatment ().
Table II. Predictor variables and hazard ratio in Final Cox Proportional Model for melanoma-associated mortality.
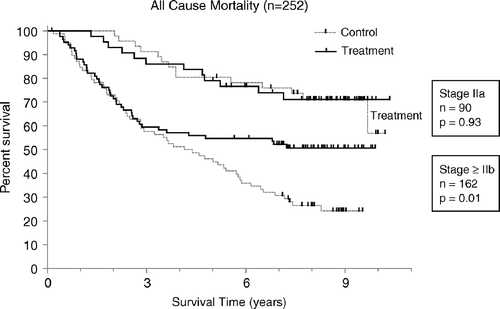
The interaction factor between stage IIa and treatment assigned was not significant and was not selected in the Cox proportional model (p > 0.68). This indicated that no significant contrast in the effect of treatment on overall survival in the subsets of patients with low-risk and high-risk melanoma (i.e. stage IIa or non-stage IIa) could be identified. The log rank p-values for the stage IIa patients (HR 0.97, CI 0.45–2.10, p = 0.937) and for the patients in non-IIa stages (HR 0.60, CI 0.40–0.90, p = 0.013) were however, markedly different ().
Figure 4. Kaplan-Meier functions of overall survival in the stage IIa population (n = 90) and the non-stage IIa population (n = 162) including 2 non-melanoma patients and 4 stage IV patients. The results show no contrast interaction of stage vs. treatment in Cox proportional hazard modeling (p > 0.68).
Salvage therapy
Following relapse, the patients received various salvage treatment regimens including additional surgery, radiation therapy, chemotherapy, immunotherapy (excluding interferon), hormone therapy and autologous tumour vaccination as single or combination approaches. Twenty-eight different regimens for salvage therapy were used, with no preponderance of any particular type of treatment in any one of the groups of treated or untreated patients. Although salvage therapy using interferon was not used in the patients who had a relapse during study follow-up, most of the patients who withdrew early from arm B went on to receive recombinant rIFN-α2 after they had withdrawn.
Safety
In total, 716 adverse events (AEs) of all WHO grades were reported in arm A patients of which 235 occurred during the period of DTIC treatment (32.8%) while 482 (67.2%) were documented during HuIFN-αLe treatment. During DTIC treatment 3.4% of all AEs were of WHO grade III/IV, while during HuIFN-αLe 2.9% had grade III/IV severity.
Most patients in arm A developed fever (64%), malaise (62%), anorexia (62%) and nausea (55%) predominantly during the initial phases of either DTIC- or HuIFN-αLe treatment. Only in 13 patients did these symptoms exceed WHO grade II. Somnolence, diarrhea and skin disorders (mainly alopecia) occurred in approximately 20–25% of patients in arm A while other events were less common.
Eleven serious adverse events (SAEs) occurred during DTIC application in arm A. Most of these were characteristic of DTIC and were treatment-related (i.e., eosinophilia, thrombocytopenia, infection, bloody diarrhoea, and elevated liver enzymes).
Besides patient death, a total of 22 SAEs occurred during treatment with HuIFN-αLe in arm A and the relationship to treatment was classified as “probable” in 14 of these 22. Eight SAEs included anorexia with “probable” or “possible” relationship to the study drug. One patient experienced impotence, which resolved after the scheduled treatment was completed. One patient suffered from injection phobia (neurosis of WHO grade III) while diarrhoea occurred in another patient. Five patients demonstrated increased liver enzymes (γ-GT, and/or AST and/or ALT) that were possibly related to treatment. In four cases the elevation in liver function parameters reached WHO grade II, in one case WHO grade IV. Application of study drug was reduced and later discontinued for one of the patients with grade 2 increased liver enzymes.
Thirteen SAEs (not including deaths) occurred during the post-treatment follow-up in am A. Three patients developed arthrosis, six patients developed neoplasms, one patient demonstrated herpes zoster, one had pneumonia and one showed increase of γ-GT. All of these SAEs were classified as not related to study treatment. One patient had amnesia (WHO grade II) with a probable relationship to treatment.
Five SAEs (not including deaths) occurred during the first two months of the study in arm B (i.e. the period of time corresponding to the DTIC treatment period in study arm A). These included: second malignant melanoma, increased LDH and increased hepatic enzymes (WHO grade III); lipoma (WHO grade II, not related) and increased γ-GT (WHO grade IV, not related). A total of six SAEs (not including deaths) were documented in study arm B during the 3 to 8-month follow-up observation period (i.e. the period of time corresponding to the HuIFN-αLe treatment period in the adjuvant treated group). These events included secondary malignant melanoma, and renal carcinoma (WHO grade III); rectal carcinoma, myocarditis, and neuralgia (WHO grade II, not related); and diabetes mellitus (WHO grade I, no relationship). No SAEs were recorded after the first eight months after randomization.
In addition to recording toxicity data by WHO scores, tolerability of the medication was assessed by the patients during the entire treatment period. Eighty-five percent of the patients characterised the tolerability as good or very good and only 3.5% characterised it as being poor.
Antibodies to IFN-alpha
Tests for serum anti-IFN antibodies were performed in the arm A population at the end of treatment period. No IFN-binding or neutralizing antibodies were detected in any serum.
Discussion
The results of the trial strongly suggest that adjuvant treatment of cutaneous melanoma with DTIC followed by low-dose natural IFN-alpha gives a sustained long-term benefit regarding reduction of melanoma-associated mortality. In support of this suggestion the hazard ratio calculated by Cox proportional hazard modeling, adjusted for identified predictive factors, showed an almost 50% reduction of the risk of dying from the disease for the entire ITT population of 252 patients, at a high level of statistical significance (p = 0.002).
The fact that the beneficial effect was more evident when proportional hazard modelling, rather than log rank testing was employed, is probably due to the heterogeneity of the study population. The total population included a disproportionately large number of stage IIa relative to stage IIb patients, meaning that the stratum of stage IIa plus IIb patients contained a larger proportion of patients with good prognosis and fewer events than was anticipated in the calculation of the power of the study. This led to a “dilution” effect, which might be assumed to have an impact on the calculations of statistical significance by log rank tests. In agreement with this assumption there was no demonstrable effect of therapy in stage IIa patients whereas the reduction of number of deaths of all causes was substantial and highly statistically significant in the non-stage IIa melanoma group. In spite of this finding analysis of treatment effect by stage interaction factor showed no significant contrast in the effect of treatment on OS in the subsets of patients with stage IIa and those with non-stage IIa, implying that it could not be excluded that the treatment had an effect also in stage IIa patients.
The above results suggest that non-stage IIa patients, i.e. those with deep and/or metastasizing tumours, may derive considerable benefit from adjuvant treatment with the combined DTIC/HuIFN-αLe regimen. In further support of this suggestion an exploratory, not prospectively planned, analysis of the clinically highly relevant group of high-risk (stage IIb–III) melanoma showed a pronounced and significant difference (p = 0.008), with respect to OS, between the two arms of the study. Among these patients all but one were followed up for an extended period of time (median 8.5 years) and thus there was no bias caused by the high withdrawal rate with respect to mortality.
A major advantage of the adjuvant regimen used in this study is its relative lack of toxicity. The good tolerability observed contrasts to the frequent, often severe adverse effects encountered with the high-dose recombinant IFN-α2 regimens. This was not surprising in view of earlier reports showing that 3 MU three times weekly of HuIFN-αLe can usually be given for several years without any major discomfort to the patient, and that the adverse effects caused by HuIFN-αLe are equal to or less pronounced than those associated with the identical dosage of rIFN-α2 treatment Citation[18]. Furthermore, the adverse effects of DTIC, at the doses of 850 mg/m2 used in this study, are usually relatively mild. The mean total incidence of WHO grade III/IV adverse effects in the study was 0.2 per patient. This figure should be compared with those obtained in the ECOG 1686 and 1690 studies when evaluating high-dose rIFN-α2 therapy with an pr patient incidence of 1.15 and 1.8, respectively Citation[1], Citation[2].
The reasons for the beneficial effect on survival observed in this trial using a 6 month low-dose IFN-α, whereas such regimens have not been effective in most other investigations, remains to be elucidated. The positive results might be related to the characteristic of the population enrolled in this trial, the sequential use of DTIC/ HuIFN-αLe, or the pharmacodynamic properties of the mix of subtypes in HuIFN-αLe. It is noteworthy that in the initial study with high-dose rIFN-α2 that showed a significant OS benefit Citation[1], the prognosis of the patients was poor (37% 5-year survival), whereas in a later study Citation[2] that showed no OS benefit, the prognosis in the control group was much better (54% 5-year survival). In this context it is important to note that the survival curves in the control group of the present study followed kinetics that closely resemble those observed in previous studies, including the continuous increase in mortality beyond five years of observation. Thus, it seems possible that patients with a relatively good prognosis and a survival expectation above three years, notably those with local or regional metastases, are particularly amenable to adjuvant treatment with low dose HuIFN-αLe or other interferon alpha preparations.
It is not possible to determine the relative roles of HuIFN-αLe and/or DTIC or of their synergy. Although DTIC is widely used for the treatment of advanced melanoma it has not been found to be effective as an adjuvant in this disease Citation[11]. It seems possible that an initial reduction of the clinically occult tumour burden, caused by DTIC, may have paved the way for the actions of HuIFN-αLe. Reduction of tumour mass may be advantageous since it may reduce tumour-induced immuno-suppression. Recently presented in vitro data suggest that DTIC may also have boosting effects on immunological anti-tumour mechanisms by sensitizing melanoma cells to the lytic activity of cytotoxic T cells Citation[19]. On the other hand, DTIC is in itself a cytotoxic drug with immunosuppressive properties Citation[20] that might interfere with the immunologic effects of HuIFN-αLe. Although the latter possibility has not received any firm experimental support Citation[21], recently presented data suggest that simultaneous combination of low-dose rIFN-α2a and DTIC in adjuvant treatment of cutaneous melanoma is not effective, as compared to rIFN-α2a alone Citation[13]. Since sequential rather than concomitant treatment with DTIC and HuIFN-αLe was used in the present study it does not seem likely that DTIC in this case has interfered with the immunological effect of HuIFN-αLe.
The mechanisms for the beneficial effect of IFN-α in cutaneous melanoma remain unknown. HuIFN-αLe is a potent stimulator of Th1 immunity Citation[22] and may therefore enhance tumour immunity and autoimmunity. A clear correlation between induction of autoantibodies by IFN-α and a good response to treatment in malignant melanoma has recently been reported Citation[23]. Further evidence for an immunologically mediated effect by IFN-α in human melanoma can be derived from studies showing that a beneficial response to IFN-α correlates with the number of CD4 lymphocytes identified in fine-needle aspirates from melanoma metastases Citation[24]. HuIFN-αLe contains a variety of subtypes of which some have immunologic effects that are not shared by recombinant IFN-α2 preparations Citation[17] and synergy between various subtypes is known to occur. Data from experimental models of melanoma about which of the interferon alpha subtypes might be particularly well suited for the adjuvant therapy of melanoma are not available.
In summary, the adjuvant treatment regimen used in the present study was found to be well tolerated and to give beneficial effects on survival that were particularly significant in patients with deep and/or metastasizing stages of melanoma but still free from distant metastases. A subsequent clinical study with sequential DTIC plus HuIFN-αLe in adjuvant melanoma treatment should therefore focus on this category of patients.
The following co-investigators (in alphabetical order) participated in this clinical project by recruitment, treatment and follow-up of melanoma patients: PJ Frosch (Dortmund), P Kaudewitz (München), G Gross (Rostock), D Kamanabrou (Münster), B Knopf (Zwickau), L Kowalzick (Plauen), M Landthaler (Regensburg), D Nashan (Münster), E Paul (Nürnberg), RU Peter (Ulm), I Pönitzsch (Leipzig) and H Schöfer (Frankfurt).
We thank Prof. Dr Manfred Hündgen and Dr Günter Stetter for the organisational management of the study and Renate Hochdorfer (all Rentschler Biotechnology Inc., Laupheim, Germany) for her data monitoring activities. Additionally we thank A Knoll (BZT Munich, Germany) and T Archambault (Virtustat, Florida, USA) for the statistical data analysis and Drs C Garbe, J Hasford and D Schadendorf (Independent Study Review Committee) for their fundamental advice in the course of the project.
The study was funded by Viranative AB (formerly BioNative AB), Umeå, Sweden, a subsidiary of Viragen Inc, Plantation, Florida, USA. Conflict of Interest: Harald von Eick and Örjan Strannegård are both advisors to Viranative AB, Umeå, Sweden.
References
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996; 14: 7–17
- Kirkwood JM, Ibrahim J, Sondak V, Richards J, Flaherty L, Ernstoff M, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 2000; 18: 2444–58
- Kirkwood JM, Ibrahim J, Lawson DH, Atkins MB, Agarwala SS, Collins K, et al. High-dose interferon alfa-2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: Results of the Multicenter Eastern Cooperative Oncology Group Phase II Trial E2696. J Clin Oncol 2001; 19(5)1430–6
- Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of Eastern Cooperative Oncology Group and Intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res 2004; 10: 1670–7
- Creagan ET, Dalton RJ, Ahmann DL, Jung S-H, Morton RF, Langdon RM, Jr, et al. Randomized, surgical adjuvant clinical trial of recombinant interferon alfa-2a in selected patients with malignant melanoma. J Clin Oncol 1995; 13: 2776–83
- Eggermont A. Adjuvant therapy in melanoma. Eur J Cancer 2003; 5(Suppl 1)S107
- Cascinelli N, Belli F, Mackie RM, Santinami M, Bufalino R, Morabito A. Effect of long-term adjuvant therapy with interferon α-2a in patients with regional node metastases from cutaneous melanoma: A randomised trial. Lancet 2001; 358: 866–9
- Cameron DA, Cornbleet MC, Mackie RM, Hunter JAA, Gore M, Hancock B, et al. Adjuvant interferon alpha 2b in high risk melanoma–the Scottish study. Br J Cancer 2001; 84: 1146–9
- Grob JJ, Dreno B, de la Salmoniere P, Delaunay M, Cupissol D, Guillot B, et al. Randomised trial of interferon α-2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. Lancet 1998; 351: 1905–10
- Pehamberger H, Soyer HP, Steiner A, Kofler R, Binder M, Mischer P, et al. Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma. J Clin Oncol 1998; 16: 1425–9
- Eggermont A, Punt C. Does adjuvant systemic therapy with Interferon-alpha for stage II–III melanoma prolong survival?. Am J Clin Dermatol 2003; 4: 531–6
- Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A metaanalysis of the randomised trials. Cancer Treat Rev 2003; 4: 241–52
- Garbe, C, Hauschild, A, Linse, R, Dummer, A, Kapp, J, Ulrich, L, et al. Final results of two adjuvant trials of DeCOG. Proceedings of the 5th International Conference on the Adjuvant Therapy of Malignant Melanoma. 2004. pp 1–22.
- Eggermont AM, Kirkwood JM. Reevaluating the role of dacarbazine in metastatic melanoma: What have we learnt in 30 years?. Eur J Cancer 2004; 40: 1825–36
- Rudolf Z, Furlan L. Adjuvant treatment of malignant melanoma with human leukocyte interferon. Period Biol 1990; 92: 141–2
- Fierlbeck G, Schreiner T, Rassner G. Combination of highly purified human leukocyte interferon α and 13-cis-retinoic acid for the treatment of metastatic melanoma. Cancer Immunol Immunother 1995; 40: 157–64
- Foster GR, Finter NB. Are all type I interferons equivalent ?. J Viral Hepatitis 1998; 5: 143–52
- Ahrén B, Engman K, Lindblom A. Tolerance to long-term treatment of malignant midgut carcinoid with a highly purified human leukocyte α-interferon. Anticancer Res 1992; 12: 881–4
- Yang S, Haluska FG. Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fas-mediated pathways. J Immunol 2004; 172(7)4599–608
- Bruckner HW, Mokyr MB, Mitchell MS. Effect of imidazole-4-carboxamide, 5-(3,3-dimethyl-1-triazeno) on immunity in patients with malignant melanoma. Cancer Res 1974; 34: 181–3
- Konjevic G, Jovic V, Radulovic S, Jelic S, Dzodic R, Spuzic I. Therapeutic implications of the kinetics of immunomodulation during single or combined treatment of melanoma patients with dacarbazine and interferon-alpha. Neoplasma 2001; 48: 175–81
- Belardelli F, Gresser I. The neglected role of type I interferon in the T-cell response: Implications for its clinical use. Immunol Today 1996; 17: 369–72
- Gogas, H, Ioannovich, J, Frangia, K, Tsoutsos, D, Panagiotou, P, Economou, TH, et al. The prevalence of of autoantibodies in patients with high risk melanoma receiving adjuvant interferon. Proceedings of the 5th International Conference on the Adjuvant Treatment of Malignant Melanoma. 2004. pp 1–11.
- Hakansson A, Gustafsson B, Krysander L, Hakansson L. Tumour-infiltrating lymphocytes in metastatic malignant melanoma and response to interferon alpha treatment. Br J Cancer 1996; 74: 670–6