Abstract
Presently, no effective systemic therapy is available for patients with advanced hepatocellular carcinoma (aHCC). We sought to determine whether systemic treatment with pegylated liposomal doxorubicin (PLD) would yield a response rate of 20% in chemotherapy naïve patients with aHCC. The study was designed according to the phase II Gehan two-step procedure with a precision of 10%. Enrolment criteria included histological diagnosis and radiological documentation of unresectable/metastatic HCC, WHO PS 0–2, relatively normal organ function, life expectancy greater than three months, lack of cardiomyopathy and active cardiac disease NYHA ≥II. PLD (40 mg/m2 IV 1h-infusion) was administered on d1 q 4 wk and response to treatment was evaluated radiologically every 3rd cycle (WHO-criteria). Secondary endpoints included overall (OS) and progression free survival (PFS) and registration of toxicity. The median number of administered PLD cycles was 3. The best radiological response among the first 14 patients was 1 PR, 5 SD, 3 PD, and 6 NE due to progressive disease clinically (Step 1). The 15th patient did not respond to the PLD-therapy and the study was closed for accrual as the pre-planned analysis could be executed (Step 2). A response rate ≥20% could be ruled out. The median PFS and OS survival was 82 days and 130 days, respectively. Adverse events were generally mild in the subgroup of patients without signs of moderate hepatic failure at base line. Patients with WHO PS 2, liver tumour involvement >50%, bilirubin ≥34 µmol/L, albumin <33 g/L, and/or Child Pugh B were unlikely to survive >90 days. PLD can be delivered safely in patients with aHCC and no signs of moderate hepatic failure. The therapy resulted, however, in few responses or cases of disease stabilization and has thus very limited activity in aHCC. Future studies on systemic chemotherapy should focus on patients without moderate hepatic failure, with WHO PS <2, and with liver tumour involvement <50%.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide Citation[1]. Surgery is the treatment of choice for patients with localized disease. The clinical management of advanced HCC (aHCC) is, however, often complex due to underlying liver disease and poor patient performance status.
The efficacy of systemic chemotherapy in aHCC has been studied in a large number of uncontrolled and controlled trials for all major classes of anti-cancer agents, given as single drug or in combination regimens and, presently, few responses and no certain effect on survival rates have been reported Citation[2]. Following initially encouraging results Citation[3], the agent doxorubicin has been tested extensively and it is presently considered as a good control for the evaluation of new drugs. However, acute and accumulative toxicity, viz. cardiotoxicty, limits adequate dosing of this compound. New formulations of doxorubicin have, therefore, been developed to reduce toxicity and to improve anti-tumour activity. Doxorubicin HCI Pegylated Liposomal (Caelyx®; Schering-Plough, Kenilworth, NJ, USA), is a liposomal formulation of doxorubicin sterically stabilized by the grafting of segments of polyethylene glycol. These liposomes can avoid uptake by the reticuloendothelial system. Thus, the formulation has a long circulation time. The liposomes are eventually extravasated through the abnormally permeable vessels of the tumour and can, thus, theoretically deliver high levels of doxorubicin in the malignant cells without affecting normal tissue.
We sought to determine whether systemic therapy with pegylated liposomal doxorubicin (PLD) would yield a radiological response rate of 20% in 15 previously untreated patients with aHCC. Secondary endpoints included progression free survival, overall survival, and treatment-related toxicity.
Material and methods
Patients gave written informed consent before entering this study. The study protocol was approved by the local ethics committee.
Inclusion criteria for this prospective phase II study consisted of age ≥ 18 years, a histological diagnosis and radiologic documentation of inoperable aHCC with measurable lesions, a WHO performance status ≤ 2, platelets ≥ 100 X 109/L, a haemoglobin value > 90 g/L, neutrophils ≥ 1.5 X 109/L or leucocytes ≥ 3 X 109/L, a s-creatinine value < 1.5 X UNL, an INR < 1.4, a bilirubin level < 2 X UNL, the use of adequate contraception, and a life expectancy > three months. Exclusion criteria included a history of cardiomyopathy and/or active cardiac disease NYHA class ≥ II, uncontrolled infectious diseases, other malignant diseases within the last five years, pregnancy/breast-feeding, conditions which would prevent adequate information and follow-up, radiation therapy within four weeks of inclusion or to more than 1/3 of the haematopoetic reserves, and prior chemotherapy for HCC. The primary endpoint was best radiological response rate according to WHO criteria Citation[4]. Secondary endpoints included progression free survival (PFS), overall survival (OS), and toxicity.
Patient population
The mean age of the patients was 67 years. The population consisted of three women and 12 men. Body weight and BMI at study start ranged from 50 – 93 kg and 18.9 – 37.2, respectively. The WHO performance status ranged from 0 – 2; five, six, and four patients were classified as WHO 0, 1, and 2, respectively. An underlying liver disease was identified in 12 cases: one case of Alagille's syndrome, four cases of cirrhosis on the basis of alcoholism, one case of porphyria, five cases of hepatitis B and/or C, and one case of unclear liver disease/PBC. Three patients had undergone a liver transplantation and in one of these cases a combination therapy of chemotherapy/stem cell transplantation was given. Nine patients had locally advanced disease confined to the liver and six had metastatic disease, viz. lung lesions. Moderate ascites was identified in four cases at base line. More than 50% of the liver parenchyma was engaged by tumour in nine patients. Bilirubin levels at base line ranged from 10 – 45 µmol/L, albumin values from 26 – 38 g/L, haemoglobin from 90 – 161 g/L, and platelets from 99 – 437 X 109/L. The levels of leucocytes/neutrophils were normal in all cases. One patient had an INR level > 1.2 at study entry. The Child Pugh score Citation[5] of the cohort ranged from 5 – 9; seven cases were classified as Child A and eight as Child B.
Treatment schedule/evaluation
Pre-treatment evaluation included physical examination, radiological assessment within four weeks prior to start of treatment, haematology and blood chemistry 72 h prior to therapy, and ECG. The patients received a fixed dose of 40 mg/m2 of PLD as IV infusion over 1h on day 1 q 4 weeks. Prophylactic antiemetics were administered and consisted typically of IV ondansetron 8 mg and betamethasone 4 mg. Haemathology was repeated weekly during the first two cycles. Haemathology and blood chemistry were controlled prior to every PLD cycle. Patients underwent, furthermore, a physical examination and registration of adverse event according to WHO-classification prior to every new administration of PLD.
Radiological assessment was performed after cycle 3 and 6 and evaluated according to WHO criteria Citation[4]. Patients with PR or SD after six courses of therapy were followed with radiological evaluation every 3rd month during the first year and every 6th month thereafter until tumour progression.
Statistics
This phase II study was designed according to the Gehan two-step procedure Citation[6]. The lowest limit of therapeutic activity considered to be of clinical importance was 20%. The selected precision was 10%. Thus, fourteen patients were treated initially (Step 1) and the number of additional patients included was based on the number of responses in this cohort (Step 2).
Overall survival (OS) and progression-free survival (PFS) from the study inclusion date were estimated using the Kaplan–Meier actuarial method Citation[7]. The relation between OS and prognostic factors was studied with either the Mann–Whitney test Citation[8], the Jonchheere-Terpstra test Citation[9], or the Spearman rank-order correlation coefficient Citation[10]. For the multivariate modeling, forward stepwise multiple regression was used. All tests performed were two-sided, and a p-value < 0.05 was considered statistically significant.
Results
The median number of administered PLD cycles was three. Delay of treatment and reduction of dose were registered in seven and two cycles, respectively.
Best response to treatment is summarized in . The best radiological response among the first 14 patients was 1 PR, 5 SD, and 3 PD (Step 1). The 15th patient was included and he did not respond to the PLD therapy (Step 2). The study was, thus, closed for accrual as the pre-planned analysis could be executed. Six patients were not evaluated radiologically after three cycles of PLD due to clinical signs/ symptoms of progressive disease and rapid deterioration of their health. The latter group of patients was included among the individuals with radiological PD as cases of treatment failure in the ensuing analyses. Only four patients received the planned six cycles of PLD and two of these cases had PD on the 2nd radiological assessment.
Table I. Best response after 3 and 6 cycles of pegylated liposomal doxyrubicin in patients with aHCC
The overall survival and progression free survival curves are depicted in . The median OS was 130 days (IQR: 68 – 473) and the median PFS was 82 days (IQR: 65 – 198).
Figure 1. The overall survival (OS) and progression free survival (PFS) curves for patients with aHCC treated with pegylated liposomal doxorubicin.
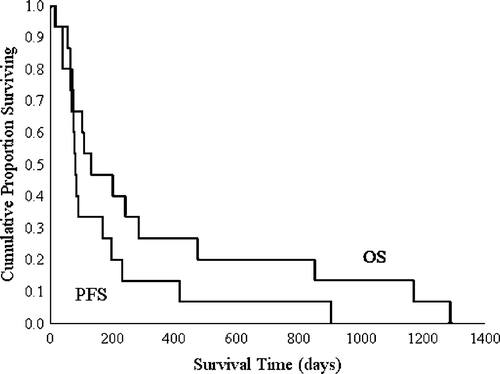
The survival of the patients was also analyzed with respect to prognostic risk factors at base line. A relation was, according to univariate analyses, found between OS and performance status (0n = 5 (median OS:851 days) vs. 1n = 6 (median OS:118 days) vs. 2n = 4 (median OS:81 days)) (p = 0.03), liver tumour involvement (median OS > 50%n = 9:72 days vs. median OS < 50%n = 5:851 days) (p = 0.003), levels of albumin (p = 0.03), values of bilirubin (p = 0.004) (level < 34n = 9 median OS:240 days vs. level ≥ 34n = 6 median OS: 65 days), and Child Pugh class, i.e. An = 7 (median OS:283 days) vs. Bn = 8 (median OS:84 days), (p = 0.02). Trends for association were found between OS and base line weight (p = 0.07), BMI (p = 0.07), and ascites (p = 0.1). No relation was evident between OS and base line levels of haemoglobin, values of platelets, levels of AFP, age, or stage of disease, i.e. patients with locally advanced tumours (median OS:130 days) vs. cases with metastatic disease (median OS:152 days).
The factors liver tumour involvement (<50% vs.>50%) (p = 0.005) and WHO PS (0 vs. 1–2) (p = 0.01) were, according to multivariate modeling, independently related to OS.
The two patients who were treated with six courses of PLD without PD were retreated when their tumours progressed. Both patients progressed after three courses when the drug was reintroduced and one of the patients died later of progressive lung metastases while the other expired in acute upper GI bleeding due to rupture of varicosed esophageal veins.
Adverse events
The side effects were generally mild in patients with good performance status at base line. Palmo-plantar erythrodysesthesia grade 1 was noticed in four cases and of grade 2 in one patient. Asymptomatic grade 3 haematological toxicity was observed during six cycles, viz. one occasion of thrombocytopenia, one incidence of leucopenia, and four observations of neutropenia. Grade 3 fatigue was registered in three patients. Dyspnoea of grade 3 was diagnosed in one case with severe chronic obstructive lung disease at base line. One patient experienced worsened ascites during therapy, which was upgraded from 2 to 3. No cardiac toxicity was registered.
Discussion
This study could not detect a response rate ≥ 20% for PLD in patients with aHCC. Furthermore, few patients experienced prolonged disease stabilization ( and ). Thus, PLD cannot be recommended as a systemic therapy in aHCC. A similarly low response rate to PLD in aHCC has also been reported by other groups Citation[11], Citation[12].
A few long-term survivors were seen in our study population (). It is not possible to conclude whether this was due to a therapeutic effect of PLD, as a wide variation in survival without treatment has been reported in aHCC.
The side effects of PLD-therapy in patients with aHCC and good performance status were minor in our treatment series. We can, however, not rule out that the treatment had a detrimental effect in patients with signs of moderate liver failure at base line, e.g. hyperbilirubinemia.
Limited survival, viz.<90 days in about 50% cases, was noted in patients with the following characteristics: Bilirubin ≥ 34 µmol/L, Child Pugh class B, albumin < 33 g/L (median value of population), WHO PS 2, and liver tumour involvement > 50%. The latter characteristics are seen in patients with a moderately compromised liver function. Neither the degree of liver tumour involvement nor the WHO PS are included in the Child Pugh scoring system, but they were both, according to the multivariate analysis in our treatment series, independently related to OS. Thus, patients with signs of moderate hepatic failure (Child Pugh B) or with WHO PS 2 or liver tumour involvement > 50% should probably not be included in future studies with chemotherapy as possible therapeutic gains are difficult to evaluate due to rapid deterioration of health/early cessation of treatment ().
Patients with metastatic disease at base line did not survive for a shorter duration than patients with locally advanced disease in our treatment series. This is peculiar to HCC and is rarely seen in other malignant tumours.
Systemic chemotherapy in aHCC has been explored extensively and presently no report supports the use of this modality outside clinical studies Citation[2]. The dismal results with chemotherapy in aHCC have raised the interest for alternative treatment strategies, e.g. targeted therapies Citation[13], Citation[14]. Early data suggest that EGFR-inhibition may benefit this group of patients Citation[15]. Also, antiangiogenetic drugs may play a role in the treatment of this disease in the future Citation[16]. A recent, promising case report has, furthermore, suggested a possible effect of mTOR inhibition on the progression of HCC Citation[17].
In conclusion, pegylated liposomal doxorubicin appeared to be an ineffective drug in aHCC with few objective responses and short duration of disease stabilization. Certain patient characteristics, viz. signs moderate liver failure, should probably be avoided in future studies on chemotherapy in aHCC. These include WHO-performance status 2, elevated bilirubin levels (>33 µmol/L), low albumin values, Child Pugh class B, and liver tumour involvement > 50%. About 50% patients with the latter characteristics did not survive 90 days and we believe that possible therapeutic gains of chemotherapy cannot be tested effectively in these subgroups due to rapid deterioration of health/early cessation of treatment. Therapies aimed at better targeting of the underlying pathological processes involved in aHCC may in the future become alternative and effective means of treating the disease.
References
- Simonetti RG, Camma C, Fiorello F, Politi F, D'Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci 1991; 36: 962–72
- Bartlett DL, Carr BI, Marsh JW. Cancer of the Liver. Cancer: Principles & Practice of Oncology, VT DeVita, Jr, S Hellman, SA Rosenberg. Lippincott Williams & Wilkins, Philadelphia 2005; 986–1008
- Ihde DC, Kane RC, Cohen MH, McIntire KR, Minna JD. Adriamycin therapy in American patients with hepatocellular carcinoma. Cancer Treat Rep 1977; 61: 1385–7
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology 1987; 7: 660–4
- Gehan EA. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. J Chronic Dis 1961; 13: 346–53
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457
- Mann H, Whitney D. On a test whether one or two random variables is stochastically larger than the other. Ann Math Stat 1947; 18: 50–60
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika 1954; 41: 133–45
- Siegel S, Castellan N., Jr. Nonparametric statistics2nd ed. McGraw-Hill, New York 1988
- Halm U, Etzrodt G, Schiefke I, Schmidt F, Witzigmann H, Mossner J, et al. A phase II study of pegylated liposomal doxorubicin for treatment of advanced hepatocellular carcinoma. Ann Oncol 2000; 11: 113–4
- Valle JW, Dangoor A, Beech J, Sherlock DJ, Lee SM, Scarffe JH, et al. Treatment of inoperable hepatocellular carcinoma with pegylated liposomal doxorubicin (PLD): Results of a phase II study. Br J Cancer 2005; 92: 628–30
- Thomas MB, Zhu AX. Hepatocellular carcinoma: The need for progress. J Clin Oncol 2005; 23: 2892–9
- Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol 2005; 23: 8093–108
- Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 2005; 23: 6657–63
- Schwartz J, Schwartz M, Lehrer D, Coll M, Kinkhabwala M, Sung M, et al. Bevacizumab in hepatocellular carcinoma (HCC) in patients without metastasis and without invasion of the portal vein. J Clin Oncol 2005;23:338a. Abstract 4122.
- Rizell M, Cahlin C, Friman S, Hafstrom L, Lonn L, Olausson M, et al. Impressive regression of primary liver cancer after treatment with sirolimus. Acta Oncol 2005; 44: 496
