Abstract
To determine the activity and toxicities of a low dose leucovorin (ldLV) plus fluorouracil (5-FU) regimen, combined with oxaliplatin administered fortnightly (modified FOLFOX-4), as a first-line therapy for patients with advanced gastric cancer. Patients were treated with cycles of oxaliplatin 85 mg/m2 on day 1 plus LV 20 mg/m2, followed by 5-FU a 400 mg/m2 bolus and a 22 hour continuous infusion of 600 mg/m2 5-FU on days 1 – 2 every two week intervals. Forty-five patients were enrolled in this study. Forty-two patients were assessable for response. One of the 42 patients demonstrated complete response, and 20 partial responses, and overall response rate of 50%. The median time to progression and overall survival time were 7.7 months (95% CI: 3.6 – 11.9 months) and 11.2 months (95% CI: 9.1 – 13.3 months), respectively. Major hematologic toxicities included grade 1 – 2 anemia (39.7%), neutropenia (30.4%) and grade 3 – 4 neutropenia (10.9%). Twelve cycles were associated with neutropenic fever. The most common non-hematological toxicities were grade 2 nausea/vomiting (20%). There was no treatment related death. The modified FOLFOX-4 regimen was found to be a safe and effective first line therapy in advanced gastric cancer.
Excepting only lung cancer, gastric cancer is the leading cause of death due to cancer worldwide Citation[1]. Despite the development of early gastric cancer detection programs, only about 30% of patients are operable at presentation, and the incidence of relapse is high. In general the prognosis is poor, and the five year overall survival of patients with advanced gastric cancer is only 5% to 15% Citation[2].
Five-Fluorouracil (5-FU) and platinum based regimens have been suggested as effective first line treatments for advanced gastric cancer. The response rate of advanced or metastatic gastric cancer to such first line therapies is usually about 30 ∼ 55% Citation[3–5]. Recently, some studies have reported that the addition of another drug to 5-FU and platinum based regimens, for example epirubicin or irinotecan, or changing cisplatin to 3rd generation platinum (oxaliplatin) increases efficacy and reduces toxicity.
This phase II study was designed to assess the safety and efficacy of oxaliplatin with low dose leucovorin (ldLV) and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) as a first line therapy for patients with advanced or metastatic gastric cancer.
Patients and method
Eligibility
Patients must have had histologically confirmed adenocarcinoma of stomach and at least one measurable lesion. Patients that had undergone previous adjuvant chemotherapy were eligible if they had completed treatment more than six months before enrollment in this study. In addition, patients had to have no central nervous system metastases; no active infection; no serious or uncontrolled concurrent medical illness; no history of other malignancies; sufficient hepatic, renal and bone marrow functions; an Eastern Cooperative Oncology Group (ECOG) performance status of 0 – 2; and an age exceeding 18 years. Patients were required to provide written informed consent to participate in the study.
Treatment protocol and dose modification
On day 1, oxaliplatin (85 mg/m2) was administered by intravenous (i.v.) infusion in 500 ml of normal saline or dextrose over 2 hours. On days 1 and 2, ldLV (20 mg/m2) was administered as an i.v. bolus, immediately followed by 5-FU (400 mg/m2) given as a 10 min i.v. bolus, followed by 5-FU (600 mg/m2) as a continuous 22 hour infusion, with a light shield. Dose modifications to oxaliplatin or 5-FU were made for hematologic, gastrointestinal, or neurologic toxicities based on the most severe grade of toxicity that had occurred during the previous cycle. Patients were assessed before starting each 2-week cycle using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC), except in the case of neurotoxicity, in which case, an oxaliplatin-specific scale was used: grade 1, paresthesias or dysesthesias of short duration, but resolving before the next cycle; grade 2, paresthesias persisting between cycles (2 weeks); and grade 3, paresthesias interfering with function. Treatment was delayed for up to two weeks if symptomatic toxicity persisted, if absolute neutrophil numbers were < 1 500 /µl or if platelet counts were < 100 000 /µl. The 5-FU dose was reduced by 25% for subsequent courses if NCI-CTC grade 3 diarrhea, stomatitis, or dermatitis had occurred, and the dose of oxaliplatin was reduced by 25% in subsequent cycles if persistent paresthesias between cycles or paresthesias with functional impairment lasting more than seven days had occurred. Treatment was continued until signs of disease progression or unacceptable toxic effects developed, or a patient refused further treatment.
Follow-up evaluation and assessment of response
Before each treatment course, a physical examination, routine hematologic studies, blood chemistry, and chest x-ray were performed. CT scans were performed to define the extent of disease and response after three cycles of chemotherapy, or sooner in there was evidence of any clinical deterioration.
Response was assessed using WHO criteria Citation[6]. Complete response (CR) was defined as the disappearance of all evidence of disease and the normalization of tumor markers for a period of at least four weeks. Partial response (PR) was defined as a ≥50% decrease in bidimensional tumor measurements, without the appearance of any new lesion or the progression of any existing lesion. Progressive disease (PD) was defined as any of the following: (1) 25% increase in the sum of the products of all measurable lesions, (2) the appearance of any new lesion, or (3) the reappearance of any lesion that had previously disappeared. Stable disease (SD) was defined as a tumor response not meeting the criteria for CR, PR, or PD. Dose intensity (mg/m2/week) was calculated as the total cumulative dose divided by the duration of treatment. Relative dose-intensity (RDI) was calculated by dividing the dose-intensity by the planned dose-intensity, and is expressed as a percentage. Planned dose-intensities, expressed as mg/m2/week, were 1000 for 5-FU and 42.5 for oxaliplatin.
Statistical methods
This trial was designed to detect a response rate of 40% as compared to a minimal, clinically meaningful response rate of 20%. A two-stage optimal design as proposed by Simon was adopted Citation[7], with a statistical power of 80% to accept the hypothesis and 4% significance to reject the hypothesis. Allowing for a follow-up loss rate of up to 10%, the total sample size required was 40 patients with measurable disease. The variables included in this study were; gender, age, previous adjuvant chemotherapy, and base line carcinoembryonic antigen (CEA) levels. Response rates with respect to the different variables were compared using Fisher's exact test. Time to progression (TTP) and overall survival (OS) were calculated using the Kaplan-Meier method. TTP was calculated from the date therapy started to the date of disease progression, death, or last follow-up. OS was calculated from the date therapy started to the date of death or last follow-up. All data were analyzed using SPSS software (version 11.0, Chicago-IL).
Results
Patients characteristics
Between January 2003 and January 2005, 45 patients were assigned for treatment at the Department of Internal Medicine at the Dong-A University Medical Center, Busan, Korea. Patient basal characteristics are listed in . The male to female ratio was 30:15, and median patient age was 56 (range 31 – 76) years. Twenty-three patients were newly diagnosed and 22 patients had recurrent gastric cancer.
Table I. Patient characteristics.
Response
Forty-two patients were assessable for response. Three patients were lost to follow-up after the first cycle. The response rate on intent to treat basis for all 45 patients was 46.6%. According to per protocol analysis the response rate was 50% (95% CI, 34.2 – 65.7%). One CR (2.4%) and 20 PR (47.6%) occurred, nine patients (21.4%) had stable disease, and 12 patients (28.6%) progressive disease. A total of 27 patients received second and third line chemotherapy; 11 patients were treated with irinotecan, ten with capecitabine, and six received taxane based salvage chemotherapy.
Survival and prognostic factor
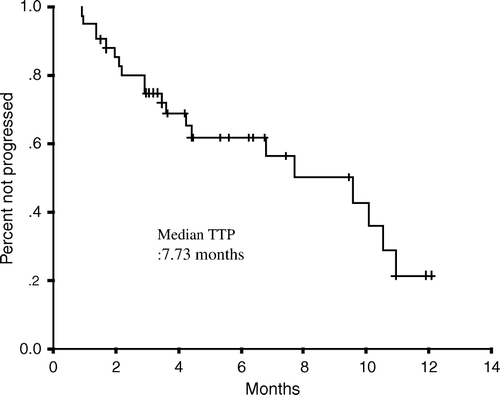
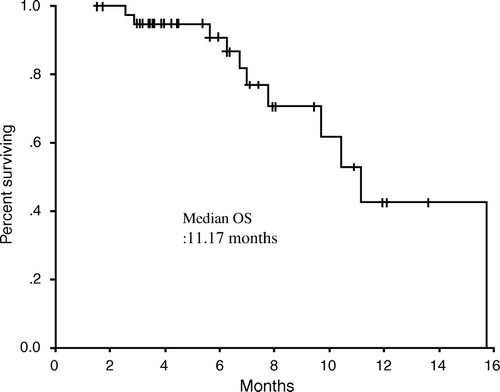
The median duration of follow-up was 10.5 (range 3.4 – 31.6) months. Median TTP duration was 7.7 (95% CI, 3.6 – 11.9) months, and median OS duration was 11.2 (95% CI: 9.1 – 13.3) months. TTP and OS were assessed by Kaplan-Meier analysis as shown in and . The one-year survival rate was 41.3%.
Analyzed factors and results are listed in . Patients with an age of < 60 years and a normal CEA showed longer TTP duration by multivariate analysis, and a normal CEA level was found to predict a longer OS duration by univariate analysis. No differences in response rates were observed with respect to the other factors e.g. number of organ involvement, hemoglobin level, palliative surgery and pervious adjuvant chemotherapy.
Table II. Toxicity of treatment.
Toxicity
Forty-five patients received a total of 249 treatment cycles. The median number of administered modified FOLFOX-4 cycles was five (range 1 – 12 cycles). The median cumulative doses were; oxaliplatin 451 (range 85 – 1020) mg/m2, LV 110 (range 20 – 2400) mg/m2, and 5-FU 10 600 (range 2 000 – 24 000) mg/m2. Dose reductions were required nine times. The dose intensities of oxaliplatin and 5-FU were 40.6 mg/m2/week and 976.5 mg/m2/week, and the RDIs of oxaliplatin and 5-FU were 95.5% and 97.7%.
Toxicities observed during the treatment are listed in . Grade I or II anemia was the most commonly observed hematological toxicity, and Grade III or IV neutropenia occurred in 10.9% of cycles. Twelve cycles (4.8%) of febrile neutropenia were recorded, and Grade I or II nausea/vomiting was observed in nine patients (20%). Grade II and III diarrhea were observed in one patient apiece. Three patients (6.7%) experienced grade III mucositis. Neuropathy was generally mild, and six patients developed Grade I or II neuropathy (6.7% and 6.7%), but no patient experienced neuropathy of grade III or more. No treatment related death occurred.
Table III. Prognostic factors.
Discussion
Most combination chemotherapy regimens for advanced gastric cancer have shown overall response rates in the range 30 to 50% in phase II studies Citation[8], Citation[9]. However, despite the introductions of new agents, such as, paclitaxel, docetaxel, irinotecan, capecitabine, TS-1, and oxaliplatin median survival has remained substantially unchanged in gastric cancer Citation[10–15].
Oxaliplatin 5-FU/folinic acid/oxaliplatin regimens have mainly been explored in colorectal cancer, and have shown encouraging activity. This combination has also been evaluated in a number of phase II studies as both a first and second line treatment for advanced gastric cancer Citation[16], Citation[17]. Reported overall response rates lie between 43 and 56%, with median survival durations of between 8.6 and 10 months, which were comparable with results reported by studies of FAMTX, ECF and ELF Citation[18–20]. Recently, the results of a first line FOLFOX-4 treatment for advanced gastric cancer were released Citation[21], and they showed a 38% overall response, as 7.1 month TTP, and an 11.2 month OS. These findings are consistent with those of previous studies, but less myelosuppressive and peripheral neuropathy toxicity were reported than for FOLFOX-6.
In the present study, we administered the combination ldLV (20mg/m2, 5-FU and oxaliplatin as first line treatment for gastric cancer. The role of LV is unclear; however, in a study of colorectal cancer, ldLV showed the same efficacy and toxicity as classically dosed LV (200 mg/m2) Citation[22], Citation[23].
Our results showed that overall response rate, TTP, and OS were 50%, 7.7 months and 11.2 months, respectively. Compared with FOLFOX-6 studies, our modified FOLFOX-4 regimen achieved a similar efficacy with median low accumulating doses of oxaliplatin, LV, and 5-FU. In addition, as compared with the FOLFOX-4 study, we achieved favorable cytopenia, neuropathy, mucositis, and diarrhea using low dose LV (). Lower incidence of grade 2∼3 neuropathy was observed. This may be due to that only 5 cycles (median) was administered. Our findings indicate that our modified FOLFOX-4 regimen had comparable efficacy and toxicities but with an easier, less expensive treatment schedule. Furthermore recently introduced short time of oxaliplatin infusion method could make it more convenient treatment Citation[24].
Table IV. Comparison of Efficacy and Toxicity of 1st line FOLFOX in AGC.
The response rates, TTP, OS, and acceptable toxicity profiles achieved by the modified FOLFOX-4 regimen demonstrate that it offers an active and well-tolerated treatment as a first line therapy in advanced gastric cancer patients. We recommend that the described modified FOLFOX-4 regimen be subject to phase III study.
Recently phase III studies were conducted on a three drug combination (docetaxel combined with cisplatin and 5-FUl) and a platinum free regimen (irinotecan (CPT-11) + 5FU/folinic acid, FORFIRI). However, TTP and OS results were disappointing versus the 5-FU and cisplatin, although the FORFIRI regimen had a reduced toxic profile Citation[25], Citation[26].
Thus, we conclude that new treatments and strategies are required to further increase survival and reduce therapeutic toxicities in advanced gastric cancer.
This research paper was supported by the Dong-A University Research Fund in 2005.
References
- International Agency for Research on Cancer (IARC), 2000.
- Ho D. Epidemiologic studies in gastric cancer. Gastric Cancer, D. Ho. Churchill Livingstone, New York 1988; 1–25
- Epelbaum R, Haim N, Stein M, Cohen Y, Robinson E. Treatment of advanced gastric cancer with DDP (cisplatin), adriamycin, and 5-fluorouracil (DAF). Oncology 1987; 44: 201–6
- Schnitzler G, Queisser W, Heim ME, Konig H, Katz R, Fritze D, et al. Phase III study of 5-FU and carmustine versus 5-FU, carmustine and doxorubicin in advanced gastric cancer. Cancer Treat Rep 1986; 70: 477–9
- Lopez M, Di Lauro L, Papaldo P, Conti EM. Treatment of advanced measurable gastric carcinoma with 5-fluorouracil, adriamycin, and BCNU. Oncology 1986; 43: 288–91
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10
- Douglass HO, Jr, Lavin PT, Goudsmit A, Klaassen DJ, Paul AR. An Eastern Cooperative Oncology Group evaluation of combinations of methyl-CCNU, mitomycin C, Adriamycin, and 5-fluorouracil in advanced measurable gastric cancer (EST 2277). J Clin Oncol 1984; 2: 1372–81
- Levi JA, Fox RM, Tattersall MH, Woods RL, Thomson D, Gill G. Analysis of a prospectively randomized comparison of doxorubicin versus 5-fluorouracil, doxorubicin, and BCNU in advanced gastric cancer: implications for future studies. J Clin Oncol 1986; 4: 1348–55
- Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer 1999; 85: 295–301
- Roth AD, Maibach R, Fazio N, Sessa C, Stupp R, Morant R, et al. 5-Fluorouracil as protracted continuous intravenous infusion can be added to full-dose docetaxel (Taxotere)- cisplatin in advanced gastric carcinoma: A phase I-II trial. Ann Oncol 2004; 15: 759–64
- Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, et al. Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 1999; 17: 319–23
- Souglakos J, Syrigos K, Potamianou A, Polyzos A, Boukovinas I, Androulakis N, et al. Combination of irinotecan (CPT-11) plus oxaliplatin (L-OHP) as first-line treatment in locally advanced or metastatic gastric cancer: A multicentre phase II trial. Ann Oncol 2004; 15: 1204–9
- Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 2002; 13: 1893–8
- Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer 2003; 89: 2207–12
- Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 2002; 20: 4543–8
- Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 2004; 91: 453–8
- Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 2000; 18: 2648–57
- Findlay M, Hill A, Cunningham D, Norman A, Nicolson M, Ford H, et al. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol 1994; 5: 609–16
- Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002; 20: 1996–2004
- De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infu sional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 2005; 92: 1644–9
- Ducreaux, M, Bouche, O, Pignon, JP, Raoul, JL, Mousseau, M, Deguiral, P, et al. Randomized trial comparing three different schedules of infusional 5FU and raltitrexed alone in first line metastatic colorectal cancer. Final results of the Federation Francophone de Cancerologie Digestive 9601 trial. Ann Oncol 2002;13:71, (abstr 259).
- Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol 2005; 23: 8671–8
- Pfeiffer P, Hahn P, Jensen HA. Short-time infusion of oxaliplatin (Eloxatin) in combination with capecitabine (Xeloda) in patients with advanced colorectal cancer. Acta Oncol 2003; 42: 832–6
- Moiseyenko, VM, Ajani, J, Tjulandin, SA, Majlis, A, Constenla, M, Boni, C, et al. Final results of a randomized controlled phase III trial (TAX 325) comparing docetaxel (T) combined with cisplatin (C) and 5-fluorouracil (F) to CF in patients (pts) with metastatic gastric adenocarcinoma (MGC). Proc Am Soc Clin Oncol 2005;23:308s. (abstr 4002).
- Dank, M, Zaluski, J, Barone, C, Valvere, V, Peschel, C, Wenczl, M, et al. Randomized phase 3 trial of irinotecan (CPT-11) + 5FU/folinic acid (FA) vs CDDP + 5FU in 1st-line advanced gastric cancer patients. Proc Am Soc Clin Oncol 2005;23:308s. (abstr 4003).