To the Editor
Combination chemotherapy is the most effective therapy in order to prolong survival and increase quality of life in patients with advanced or metastatic gastric cancer Citation[1–3] and renders consistently survival benefit when compared with best supportive care, achieving moderate response rates, in the range of 15–50% Citation[1], Citation[3], Citation[4]. At the present, the median survival of patients with metastatic disease remains between six and nine months Citation[1], Citation[3], Citation[4] and no regimen has demonstrated superiority Citation[5].
Kim et al. Citation[6] conducted a phase II trial with a combination of cisplatin 60 mg/m2 on day 1 and capecitabine 1250 mg/m2 twice daily days 1–14 every three weeks. They achieved an overall response rate (ORR) of 55% and the median overall survival (OS) was 10.1 months, with an acceptable toxicity. To this promising results must be added the outstanding convenience of this regimen: since there is no need for catheters or pumps for continuous chemotherapy infusion, it lessens the number of visits, the morbidity and the inconvenience of other combinations. However, we wanted to find out to what extent these results could be obtained in our daily practice. Patients with metastatic gastric adenocarcinoma usually present poor general condition, under nourishment, advanced age, and co-morbidity. These features can determine worse prognosis, lower response rate, shorter survival and increased toxicity, although do not formally contraindicate chemotherapy. Thus, increasing quality of life, lessening toxicity and considering patients′ convenience are a major concern, since advanced gastric cancer treatment is directed toward life extension and symptom palliation rather than cure.
From March 2003 to September 2004, 36 patients with ECOG performance status 0–2 and histologically confirmed, non-resectable gastric adenocarcinoma or adenocarcinoma of the gastroesophageal junction were enrolled. Their characteristics are listed in . All patients gave written informed consent as approved by the Ethical Committee. A total of 140 cycles were administered. The mean number of cycles per patient was 3.9 (range: 1–8). Delays and dose reductions were mainly due to hematologic and gastrointestinal toxicity. The dose intensity was 17.6 mg/m2/week for cisplatin and 9729 mg/m2/week for capecitabine (88% and 83% of the planned dose respectively).
Table I. Patients′ characteristics
All patients were assessed for toxicity according to NCI-CTC v2.0. The incidence of grade 3–4 diarrhea was 25%, while 14% of patients presented grade 3–4 mucositis and only 8% of them suffered grade 3–4 emesis (very light, probably due to the relative low dose of cisplatin). Despite the high doses of capecitabine administered only 8% experienced grade 3 hand-foot syndrome, dose limiting toxicity of capecitabine in phase I trials Citation[7]. Hematological toxicity predominated: 19% of patients presented grade 3–4 neutropenia while 5% experienced grade 3 anemia and 8% grade 3 thrombocytopenia. There was a high incidence of grade 4 febrile neutropenia, four patients (11%), two of them with fast recovery and the other two resulting in toxic deaths. Another toxic death occurred in a patient who developed acute renal failure due to grade 4 diarrhea and vomiting. This represents a too high incidence of toxic deaths, above 20%, not admissible for a palliative treatment, and also discordant with the results of the phase II trial Citation[6]. It is impossible to find out the reason for the difference between our results regarding toxicity and those reported by Kim el al., although different population characteristics regarding age, PS and ethnic difference in susceptibility to capecitabine in our Caucasian patients can be contributing factors.
WHO criteria Citation[8] were used for assessment of response every three cycles: three complete responses (8%), eight partial responses (22%), 13 disease stabilization (36%) and six disease progression (17%). In the remaining six patients response could not be evaluated (three toxic deaths after first or second cycle and three due to early withdrawal). Confirmed clinical benefit (defined as CR, PR, or SD) was observed in 24 (67%) of 36 patients. ORR was 30% (95% CI 15–45%) on an intention-to-treat analysis, that is, lower than in the aforementioned trial by Kim et al. (55%), but similar to those obtained with cisplatin plus 5-FU or other doublets, ranging between 20% and 60% in phase II trials Citation[9–13].
Overall survival (OS) and progression free survival (PFS) were estimated using the Kaplan-Meier method. Survival curves were compared with the log-rank test. Median OS was 7.3 months (95% CI 4.8–9.8 months). One-year survival was 20%. Median PFS was 6.8 months (95% CI 6.6–7.1 months). Again, these results were worse than those from Kim et al. (median OS 10.1 months) but similar to those obtained with combination therapy (median OS 7–9 months).
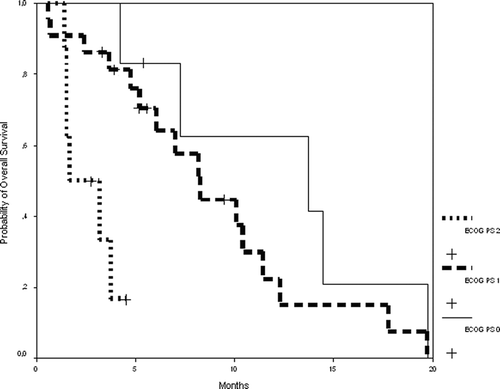
Our patients showed worse baseline conditions that could have influenced these results. No patient was excluded on the basis of advanced age: our patients had a median age of 60 years old (range 29–79), with 15 patients (42%) 65 or older, and seven (19%) older than 70. Patients at this age are usually under represented in (or excluded from) clinical trials, so results of efficacy and toxicity to compare with are lacking Citation[14]. One third of the patients had two metastatic locations and another quarter had three or more (instead of 7%), indicating a high tumor burden. Other baseline conditions related to nutritional and functional status were also unfavourable, since 22% of the patients had their albumin under normal level, 72% had significant anemia, and 33% had a weight loss of more than 10 kg. Moreover, 17% of our patients were not chemo-naive since they had received adjuvant 5-FU based chemotherapy. Finally 22% had ECOG PS 2 (instead of 7%), which in our series correlates with shorter overall survival: median OS was 13.7 months for patients with ECOG PS 0, whereas it was 8.3 months for patients with ECOG PS 1, and 1.7 months for patients with ECOG PS 2 (p = 0.0007) ().
In our series ECOG PS and administration of second-line chemotherapy were the only statistically significant factors influencing overall survival, thus indicating that patient's functional status is of major importance for predicting the outcome and selecting patients. However, number of metastatic locations, a factor related with tumor burden, was not related with survival. Neither were tumor location, sex, and previous adjuvant treatment with chemotherapy. Moreover, as stated above no differences in responses were seen between patients having received or not previous 5-FU based adjuvant chemotherapy (χ2 1.093, p = 0.618). Confirming our findings that this treatment is active in previously pretreated patients, there has recently been published a phase II trial with the same schedule in patients relapsing after fluoropyrimidine-based adjuvant chemotherapy. Authors report an overall response rate of 28%, with a median time to progression of 5.8 months and median overall survival of 11.2 months Citation[15].
In conclusion, cisplatin and capecitabine in combination are active in non-selected patients with advanced gastric cancer. Although this schedule has not been directly compared with protracted 5-FU infusion, it offers a good convenience avoiding the need of devices for parenteral infusion and with the feasibility of oral delivery. However, in our population it showed an unacceptable toxicity, with three toxic deaths although the adverse effects were otherwise mild.
A recent phase III trial has shown the superiority of triplet (including a taxane), at the expense of higher toxicity Citation[16]. Nowadays medical oncologists have at our disposal a wide range of possibilities when choosing a regime for each patient. We consider that this offers us the possibility to customize chemotherapy. For young, healthy patients with a good performance status and without relevant co-morbidity triplets may be a good option. However, for older patients with poor general condition and concurrent medical problems, not able to cope with combination chemotherapy, monotherapy or even best supportive care should be considered in order to lessen toxicity.
This study was partially reported in ICACT 2005.
References
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, doxorrubicin and methotrexate (FAMTX) plus supportive care versus supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995; 71: 587–91
- Scheithauer W, Kornek G, Zeh B, et al. Palliative chemotherapy versus supportive care in patients with metastatic gastric cancer: A randomized trial. Proceedings of the Second International Conference on Biology, Prevention and Treatment of GI Malignancy. Germany, Köln 1995; 68
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8: 163–8
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993; 72: 37–41
- Wöhrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol 2004; 15: 1585–95
- Kim TW, Kang YK, Ahn JH, Chang HM, Yook JH, Oh ST, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. Ann Oncol 2002; 13: 1893–8
- Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, et al. Preliminary studies of a novel oral fluoropyrimidine carbamate: Capecitabine. J Clin Oncol 1998; 16: 1795–802
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Lacave AJ, Baron FJ, Anton LM, Estrada E, De Sande LM, Palacio I, et al. Combination chemotherapy with cisplatin and 5-fluorouracil 5-day infusion in the therapy of advanced gastric cancer: A phase II trial. Ann Oncol 1991; 2: 751–4
- Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J, et al. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factors analysis. Eur J Cancer 1994; 30A: 1263–9
- Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorrubicin versus etoposide, leucovorin and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 2000; 18: 2648–57
- Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorrubicin and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 1993; 71: 3813–8
- Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil plus tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group study (JCOG9205). J Clin Oncol 2003; 21: 54–9
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Eng J Med 1999; 341: 2061–7
- Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH, et al. Phase II study of capecitabine and cisplatin as first-line combination therapy in patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. Br J Cancer 2005; 92: 246–51
- Moiseyenko VM, Ajani J, Tjulandin SA, Majlis A, Constenla M, Boni C, et al. Final results of a randomized controlled phase III trial (TAX 325) comparing docetaxel (T) combined with cisplatin (C) and 5-fluorouracil (F) to CF in patients (pts) with metastatic gastric adenocarcinoma. Proc Am Soc Clin Oncol 2005; 23: 4002