Abstract
Background. Aging is associated with an increasing of comorbidity and at the time of lung cancer diagnosis patients present one or more other serious disease. The aim of the study is to evaluate tolerability, response and survival of weekly paclitaxel in elderly patients with advanced NSCLC and concomitant diseases. Methods. Patients with advanced NSCLC who were poor candidates to platinum-based therapy because of age >65 years and coexistent illnesses received weekly paclitaxel over 1 hour at a dose of 80 mg/m2. Comorbidity was evaluated according to the Charlson scale, Kaplan-Feinstein index and the Cumulative Illness Rating Scale. Results. A total of 57 patients (median age, 74 years; range, 65–84) were included. The overall response rate was 44%. Median survival was 7.8 months. Grade 3–4 toxicity was uncommon: neutropenia 1.8%, thrombocytopenia 1.8%, neuropathy 7%, hypersensitivity reaction 1.8%. Comorbidity indexes were useful to characterize better the population of elderly patients, but did not define a subgroup with worse prognosis. Conclusions. The low toxicity profile and efficacy of low-dose weekly paclitaxel justified its usage in this group of poor prognosis elderly patients with advanced NSCLC and comorbidities. A comorbidity index should be introduced in prospective oncological studies in the elderly to ensure compatibility.
Lung cancer is predominantly a disease of the elderly with more than 50% of all cases occurring in people older than 65 years and about 30% over the age of 70 years Citation[1]. Demographic analysis shows an expansion of the older population and thus an increase of the number of elderly patients with lung cancer for the future.
Aging is associated with an increasing prevalence of diseases and disabilities, which often multiple. These co-morbidities and decreased functional reserve in older patients may have a negative impact on the toxicity degree and survival. In addition, the shortened life expectancy of these frail patients limits survival benefit from aggressive therapy. Although, platinum-based chemotherapy has become the standard of treatment for patients with advanced non-small cell lung cancer (NSCLC) since the meta-analyses of randomised studies that compared chemotherapy with best supportive care Citation[2–4], most of the frail patients are not suitable for this kind of aggressive therapy. The management of this population implies a delicate balance between the risks and the complications of disease and the risks and the complications of treatment and represents a novel and major challenge to medical practice.
Among the elderly population a higher degree of heterogeneity compared to the younger groups can be observed. This heterogeneity is predominantly caused by changes in functional status due to age and the presence of co-morbidities and the severity of them. Efforts should be addressed in two directions: first, to develop chemotherapy schemes with low toxicity aimed to this kind of patients; second, to characterize better the population of “frail patients” included in the studies to do they comparables.
Although, some controversy exists related to the treatment of elderly patients with combination chemotherapy or with monotherapy, ASCO guidelines recommend single agent therapy in elderly patients and patients who have a poor performance status Citation[5]. Paclitaxel has been demonstrated to have significant activity in chemotherapy-naïve NSCLC patients with responses ranging from 10 to 38% and median survival ranging from 6 to 11 months Citation[6]. Several different administration schedules of paclitaxel have been used including 24 h, 3 h and 1 h infusions in addition to weekly dosing. The commonly used schedule of three-hour paclitaxel 175 mg/m2 every three weeks (with or without carboplatin) is associated with moderate or severe neutropenia. More frequent dosing schedules of this phase-specific agent, such as weekly administration, may offer a theoretical advantage in terms of cytotoxicity due to prolonged cellular exposure to paclitaxel with diminished toxicity Citation[7]. In a phase II study, we demonstrated that low-dose of weekly paclitaxel (80 mg/m2) is effective in pretreated NSCLC patients with a response rate of 37.5% and median overall survival of 9.7 months. No grade 3 or 4 neutropenia, anaemia or thrombocytopenia was observed. Non-haematological toxicities were also mild: 7.5% presented grade 3 fatigue and 10% grade 3 motor neuropathy Citation[8]. The activity of weekly paclitaxel and low toxicity profile prompt us to test this schedule in elderly patients with NSCLC and co-existent clinical conditions that precludes further aggressive combination therapy.
The definition of “frail patients” should be clarified in order to compare these populations from different trials. Co-morbidity indexes are a way to substitute the number of co-morbid conditions by a score that reflect the severity of the conditions and the prognosis impact of those in overall mortality. Several indexes have been introduced to rate the severity of the diseases associated in cancer patients. Three of them have the most data available in the published literature: The Charlson co-morbidity index Citation[9] is probably the most widely used co-morbidity index. Nineteen diseases are weighted from 1 to 6 according to their relative risk of death and are added to form a total score. Additionally, the Charlson can be adjusted by age. The Kaplan-Feinstein index Citation[10] consists of a list of 12 conditions that are rated from 0 to 3 according to severity. These scores are summarized in a global score from 0 to 3. In the Cumulative Illness Rating Scale (CIRS) Citation[11] the co-morbidities are classified by organ system affected and rate them according to their severity from 0 to 4 (no disease, light, moderate, severe, extremely severe/life-threatening) in a way similar to the classification of toxicity in chemotherapeutic studies. The score can be summarized into number of systems involved, total score, mean score, and number of grade 3 and 4 diseases.
As far as we known, studies specifically addressed to evaluate the tolerability of chemotherapy in elderly patients with co-morbidities have not been performed. On the basis of our previous experience with weekly paclitaxel in second line chemotherapy in patients with NSCLC Citation[8], which showed good toxicity profile and activity, we decided to assess the tolerability and efficacy of low-dose of weekly paclitaxel in the treatment of elderly patients diagnosed of advanced NSCLC with co-existent diseases contraindicating the use of cisplatin-based therapy. Assessment of co-morbidity using three different indexes was performed as part of their initial evaluation to improve characterization of the population.
Patients and methods
Eligibility
Patients with histologically- or cytologically-confirmed unresectable or metastatic NSCLC without prior therapy were eligible if they were considered poor candidates for platinum-based combination regimens because of advanced age (≥65 years) and coexistent illnesses such as chronic obstructive lung disease, vascular disease, heart disease, renal impairment, etc. Other eligibility criteria were: life expectancy >2 months; Eastern Co-operative Oncology Group (ECOG) performance status (PS) of ≤2; adequate bone marrow reserve (defined as absolute granulocyte count ≥2000/µl and platelet count ≥75 000/µl); adequate hepatic and renal function (defined as serum creatinine level ≤2 mg/dl, AST and ALT ≤ 2 times the upper limit of normal, bilirubin ≤1.5 mg/dl, alkaline phosphatase values ≤5 time the upper limit of normal). Exclusion criteria included pre-existing motor or sensory neurological symptoms >2 (NCI-CTC), symptomatic brain metastases, active infections or serious social conditions that precluded weekly attendance at the hospital.
The study was conducted in compliance with institutional review board regulations, and all patients signed informed consent prior to entry into the study.
Pre-treatment and follow-up evaluation
Pre-treatment evaluation included a complete clinical history and physical examination, complete blood cell count, serum chemistries (including liver and renal function tests and electrolytes), chest x-ray and computed tomographic (CT) scans of the chest and upper abdomen. Additional tests were performed in case of symptoms suggesting metastases at other sites.
Co-morbidity was evaluated according to the Charlson co-morbidity index Citation[9], the Kaplan-Feinstein index Citation[10] and the Cumulative Illness Rating Scale (CIRS) Citation[11]. During treatment, patient monitoring included a weekly complete blood cell count during the first four weeks. Follow-up history and physical examination, serum chemistries, tumour measurements and toxicity assessment according to the NCI-CTC scale Citation[12] were performed before each 5-week of course of therapy. For response evaluation, a CT scan was performed after 8 weeks of treatment.
Treatment schedule
Treatment consisted of paclitaxel at a dose of 80 mg/m2 administered as a 1-hour intravenous infusion at the outpatient chemotherapy clinic. Treatment was given weekly, without a rest period, until disease progression, unacceptable drug toxicity, best response had been achieved or until the attending physician/investigator considered that there was no benefit accruing from the chemotherapy. Dexamethasone (8 mg), dexchlorpheniramine (5 mg) and ranitidine (50 mg) were prescribed 30 minutes before paclitaxel infusion. Prophylactic anti-emetics were not routinely provided.
World Health Organization (WHO) response criteria Citation[13] were used for efficacy analysis. Patients who were withdrawn from trial prior to response evaluation because of rapidly progressive lung carcinoma were considered as non-responders. All patients who received at least one week of chemotherapy were evaluated for toxicity.
Statistical analysis
Simon's two-stage minimax design was used to determine sample size and decision criteria Citation[14]. It was assumed that a response rate of 25% in eligible patients would indicate potential usefulness while a rate of 10% would be the lower limit of interest with a significance level of 0.05 and a statistical power if 90%. Thirty-one patients entered in the first stage of the study. Under the null hypothesis (for response rate 10%) the probability of early termination was 62%. If three or more patients responded, the accrual was continued until 55 patients. The planned accrual period was 24 months. Secondary end points were toxicity and overall survival. Response and survival rates were both calculated on an intent-to-treat basis. Overall survival and time to progression were measured from the date of entry into the trial up to time of death or up to the date of the last follow-up clinical assessment. Time to progression and overall survival curves were constructed using Kaplan-Meier method and compared using log rank, Breslow and Tarone-Ware tests. Multivariate analysis of the effect of the different parameters on survival was performed by a Cox model.
Results
Demography
The characteristics of the 57 patients included in the study are outlined in . Only a minority of them were females. Median age was 74 (range 65 – 84), forty (70%) patients were ≥70 years and nine (16%) were ≥80 years. The majority of the patients (n = 37) presented stage IV disease (65%) and a poor performance status (PS of 2 in 61.5%). The study population showed a high prevalence of co-morbid diseases () which included altered bone marrow reserve (Hb ≤ 12.5 g/dl, platelets ≤100 000/µL, or neutrophils ≤2 000/µL), diabetes, impaired renal or hepatic function, malnutrition, alcohol abuse, hypertension, vascular disease, antecedents of other tumors and AIDS. The Charlson co-morbidity index was >2 in 27 patients (47%) and the Charlson combined score (co-morbidity + age) was >5 in 26 patients (46%) (a). In the Kaplan-Feinstein scale about 65% of patients had a score of 3 (b). Thirty one (54%) of patients had more than three categories endorsed in the CIRS-G score, total score was >6 in 28 patients (49%) (c) and the severity index (total score/total number of categories endorsed) was >1.8 in 31 patients (54%).
Figure 1. Distribution of comorbidity according to several scores: (a) Charlson Index; (b) Kaplan-Feinstein index; (c) Cumulative Illness Rating Score (CIRS-G): total score.
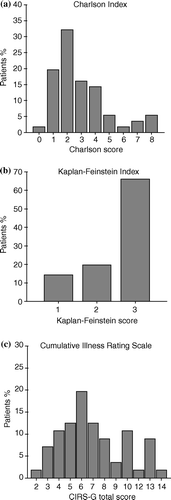
Table I. Patients' characteristics
Table II. Prevalence of comorbidities in the study population (n = 57)
Compliance and response
A total of 530 courses of weekly paclitaxel were delivered, with a median of 10 weeks per patient (range 1 to 23). Six patients received less than 4 weeks of treatment because of: hypersensitivity reaction despite pre-treatment measures (1 patient); heart attack in a patient of 84 years and rapid deterioration of PS due to progressive disease in four patients. All of them were considered as progressive disease for response analysis. Twenty-four cycles were omitted; no dose reductions were necessary.
Of the 57 patients entered into the study, 25 (44%; 95% CI, 34.3% to 53.7%) had a partial response (intent-to-treat) to weekly paclitaxel. SD was achieved in 15 patients (26%), whereas 17 patients (30%) experienced PD.
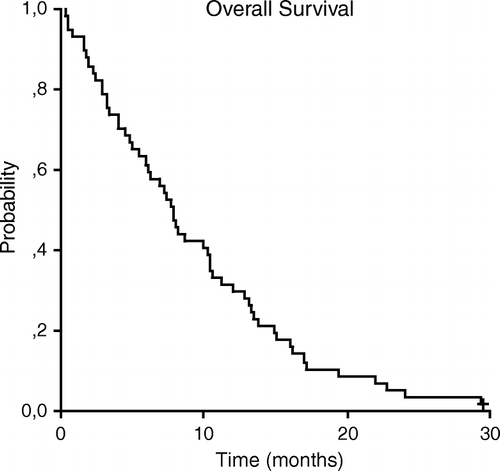
The median time to progression was 4.7 months (95% CI, 3.0 to 6.3). The median overall survival for the entire group was 7.8 months (95% CI, 6.5 to 9.1) (). The 1-year survival rate was 31%. Median survival was significantly better in patients with a PS 0–1 (10.4 months, 95% CI, 9.2 to 11.6) than in those with PS 2 (4.9 months, 95% CI, 2.3 to 7.8). No statistically significant differences were observed in median survival when the analysis was performed according to stage (III vs IV) or age (<70 vs ≥70 years). Patients with Charlson Combined Index from 0 to 5 had a median survival (8.2 months, 95% CI, 4.8 to 11.6) no statistically different than patients with score >5 (7.8 months, 95% CI, 2.8 to 11.8). Patients with Kaplan-Feinstein index of 0–2 had a better median survival (11.7 months, 95% CI, 5.6 to 17) versus those with a value of 3 (6.3 months, 95% CI, 3.5 to 9), but that not reach statistically significant difference. When we use CIRS-G scale, neither total score, nor number of categories endorsed, nor number of categories of severity 3 or 4 served to identify two groups of statistically significant differences in median survival.
Toxicity
The haematological and non-haematological toxicities are described in . In general, weekly paclitaxel was well tolerated by elderly patients in spite of severe co-morbidity. Grade 3 or 4 haematological and non-haematological toxicities are uncommon. A patient with severe chronic hepatopaty developed grade 4 neutropenia and grade 3 thrombocytopenia with slow recuperation and he was withdrawal of the study. Grade 4 non-haematological toxicity was observed in an 84 years old patient with cardiac ischemia who developed infarction after the first dose of paclitaxel. Grade 3 non-haematological toxicities were: pneumonia (one patient); hypersensitivity reaction (one patient); motor neuropathy (one patient), sensory neuropathy (three patients). Others non-haematological toxicities were mild, being fatigue and alopecia the most common: Twenty patients with grade 1–2 fatigue and 23 patients with alopecia grade 1–2.
Table III. Toxicities observed during treatment (National Cancer Institute-Common Toxicity Criteria)
Discussion
Lung cancer may be considered a disease of elderly patients. In recent years, a demographic shift towards an older population has been observed in Westerns countries and, therefore, more patients are at risk of developing lung cancer. The ASCO guidelines recommend a two-drug regimen (a platinum-based or nonplatinum combination) for advanced NSCLC patients with good performance status (i.e. PS0 or PS1) Citation[5]. However, the decision to treat or not to treat is sometimes based on the chronological age, but more frequently in the presence of co-morbid diseases and performance status. Aging is associated with increasing prevalence of co-morbid conditions Citation[15] and this may have a considerable impact in overall health and PS. Age, co-morbidity and PS are associated with increased risk of complications and poor tolerance to chemotherapy and, consequently, these patients are usually excluded from clinical trials and they received a treatment with more favourable toxicity profile, such as single agent therapy. This means that little is known about the best way to treat these patients and the best regimen for their treatment is controversial. Lilenbaum et al. Citation[16] performed subset analyses of the special population included in a randomized phase III study to compare the efficacy of combination chemotherapy with carboplatin and paclitaxel versus single agent paclitaxel. For elderly patients, the one year survival was not statistically different for combination chemotherapy or for single agent: 35% and 31%, respectively (p = 0.50). However, elderly patients included in that trial were thought to be appropriate to receive a platinum-based combination, and may not be representative of all elderly patients Citation[16]. The Multicenter Italian Lung Cancer in the Elderly Study (MILES) Citation[17] phase III randomized trial addressed to compare the effectiveness and toxicity of the combination vinorelbine plus gemcitabine with those of each drug, showed that the combination has no advantage over either single agent in the treatment of elderly patients with advance NSCLC. Median survival was similar in the three arms, whereas the combination of gemcitabine and vinorelbine resulted more toxic.
Nevertheless, in those studies selection of patients was performed according to chronological age and co-morbidities were not assessed. Generally, patients with severe co-morbidity or poor performance status were excluded of the clinical trials, for example, more than 80% of patients in the MILES study had PS 0–1 and no data of coexistent illness was reported Citation[17]. Therefore, scarcely data exist about the optimal treatment of elderly patients with severe co-morbidity. In our previous study, weekly administration of paclitaxel showed to have high activity with mild toxicity in pretreated patients with advanced NSCLC Citation[8], which make this regimen an attractive option for elderly patients. We conducted this phase II study specifically designed to elderly patients with advanced NSCLC and coexistent illnesses. Although, in clinical trials, the age of 70 is used as lower limit for patient selection, we preferred the age cut-off of 65 years because the presence of co-morbidity may lead to an increase in the number of complications during chemotherapy. In our study, weekly paclitaxel have showed good tolerability and a clinical benefit in this group of high-risk patients. An overall response rate of 44% and a median survival of 7.8 months were obtained without severe toxicity. Data from other phase II trials using other single agent chemotherapy (gemcitabine, vinorelbine, docetaxel)Citation[18] or weekly paclitaxel in the elderly Citation[19–21] have showed high variability in median survival (from 2.4 to 10.3 months) and response rate (range from 3 to 39%). Biases in patient selection rather than the drug or dose employed may explain these differences, and so, a better characterization of the population seems mandatory. Several indexes have been introduced to rate the severity of the diseases in older patients. In the present study, we have employed three of them for measuring co-morbidity in elderly patients with advance NSCLC Citation[9–11]. None of them have shown utility to identify a subgroup of patients whose co-morbidity give a worst prognosis. Neither Charlson scale nor Kaplan-Feinstein index nor CIRS-G served to predict the overall survival in this group of elderly patients with advanced NSCLC treated with single agent paclitaxel. Possible limitations of the utilization of these indexes in oncology include the fact that the indexes ignore several co-morbidities that may be relevant in designing the treatment of cancer patients. Moreover, primary alterations of physiological functions (renal function, liver metabolism, bone marrow reserve …) or age-specific phenomena (depression, alterations of metal status, reduced nutritional status, missing social support, etc.) may contribute to the tolerance of chemotherapy and the overall survival. Other reason for its limitation in this setting is that its predictive ability seems to decline progressively as the end point is shorter than 6 months, because of short-term mortality is dominated by acute events Citation[22].
In conclusion, the low toxicity profile of low-dose weekly paclitaxel suggests that this schedule would be promising in elderly patients with co-existent clinical conditions that preclude further aggressive combination therapy. In addition, the favorable response rate, median time to progression and overall survival observed (65% had stage IV and 61.5% had PS 2) justifies its usage in this group of poor prognosis patients with advanced NSCLC. Comparisons among studies in NSCLC elderly patients treated with chemotherapy are difficult because of the different selection criteria of the patients and the differences in the prevalence of co-morbidities. A good measurement and understanding of the coexistent illness and the physiological changes with the age is essential to future progress in the treatment of the elderly cancer patients. Co-morbidity is independent from functional status and therefore can provide additional prognosis information in older cancer patients. Several indexes to assess co-morbidities or functional status are available; however the applicability in geriatric oncology seems to have limitations. The development of a specific index to assess the real impact of co-morbidities and functional status in the outcome of oncological treatments are warranted.
Conflict of interest statement
The authors disclose no potential conflict of interest.
References
- Gridelli C, Perrone F, Monfardini S. Lung cancer in the elderly. Eur J Cancer 1997; 33: 2313–4
- Souquet PJ, Chauvin F, Boisel JP, Cellerino R, Cormier Y, Ganz PA, et al. Polychemotherapy in advanced non-small cell lung cancer: A meta-analysis. Lancet 1993; 342: 19–21
- Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy versus supportive care in advanced non-small cell lung cancer: Results of a meta-analysis of the literature. Chest 1994; 106: 861–5
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using update data on individual patients from 52 randomized clinical trials. Br Med J 1995;311:899–909.
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S, Jr, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J Clin Oncol 2004; 22: 330
- Socinski MA. Single-agent paclitaxel in the treatment of advanced non-small cell lung cancer. The Oncologist 1999; 4: 408–16
- Lopes NM, Adams EF, Pitts TW, Bhuyan BK. Cell kill kinetics and cell cycles effects of Taxol on human and hamster ovarian cell lines. Cancer Chemother Pharmacol 1993; 32: 235–42
- Juan O, Albert A, Ordoño F, Casany R, Carañana V, Campos JM, et al. Low-dose weekly paclitaxel as second-line treatment for advanced non-small cell luna cancer: A phase II study. Jpn J Clin Oncol 2002; 32: 449–54
- Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal stusies: Development and validation. J Chron Dis 1987; 40: 373–83
- Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. J Chron Dis 1974; 27: 387–404
- Linn Bs, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968; 6: 622–6
- Cancer Therapy evaluation program. Common toxicity Criteria, Version 2.0., DCTD, NCI, NIH, DHHS; 1998.
- WHO. WHO Handbook for Reporting Results of Cancer Treatment. Geneva: WHO; 1979.
- Simon R. Optimal two-stages design for phase II clinical trials. Control Clin Trials 1989; 10: 1–10
- Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patient. J Clin Oncol 1998; 16: 247–62
- Lilenbaum RC, Herndon JE, List MA, Desch C, Watson DM, Miller AA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The Cancer and Leukemia Group B (study 9730). J Clin Oncol 2005; 23: 190–6
- Gridelli C, Perrone F, Gallo C, Cigorali S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-sell lung cancer: The Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Nat Cancer Inst 2003; 95: 362–72
- Gridelli C, Shepherd FA. Chemotherapy for elderly patients with non-small cell lung cancer. A review of the evidence. Chest 2005; 128: 947–57
- Fidias P, Supko JG, Martins R, Boral A, Carey R, Grossbard M, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res 2001; 7: 3942–9
- Garbo L, Marsland T, Garfield D, Khan M, Asmar L. A phase II study of weekly paclitaxel (Taxol® in stage IIIB, IV or relapsed after local therapy, non-small cell lung cancer (NSCLC) patients with performance status 2 and/or ≥70 years (yrs) of age, with (Paraplatin® administered at disease progression [abstract]. Proc Am Soc Clin Oncol 2001; 20: 267b
- West W, Birch R, Sysel IA, Schell FM, Liebmann J, Tongol J, et al. A phase II trial of weekly paclitaxel in elderly patients or those with decreased performance status with advanced non-small cell lung cancer [abstract]. Proc Am Soc Clin Oncol 2001; 20: 258b
- Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol 2000; 35: 181–200