Abstract
More than two-thirds of patients with gastric cancer present with metastatic disease and their curative options are limited. This phase II study assessed the efficacy and tolerability of cisplatin, epirubicin, tegafur–uracil (UFT) and leucovorin in patients with metastatic gastric cancer (MGC). Thirty-nine patients with previously untreated metastatic or unresectable gastric cancer received intravenous cisplatin 60 mg/m2 and epirubicin 50 mg/m2 on day 1 of a 28-day cycle; UFT 300 mg/m2 was administered with oral leucovorin 30 mg/day in divided doses on days 1–22, followed by a 7-day rest. Two patients achieved a complete response, 13 had a partial response (overall response rate 38%; 95% confidence interval [CI] 24–52%) and 16 patients (41%) had stable disease. Median time to progression was 6.5 months (95% CI 5.5–7.5 months); overall survival was 9.5 months (95% CI 8.5–13.5 months). Grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 20%, 8%, and 3% of patients, respectively; two patients experienced febrile neutropenia. Grade 3 diarrhea occurred in three patients. The combination of cisplatin, epirubicin, UFT, and leucovorin has significant activity and tolerable toxicities in patients with MGC and represents a convenient treatment option for these patients.
Gastric cancer is one of the major causes of cancer death worldwide Citation[1]. Surgical resection of the tumor is the preferred option for localized disease; however, two-thirds of patients have advanced disease at presentation Citation[2]. The outlook is poor for these patients: estimated five-year relative survival in patients with stages IIIA, IIIB, and IV disease is 20%, 8%, and 7%, respectively Citation[3]. For patients for whom surgery is not an option, chemotherapy represents an effective alternative to best supportive care. Overall survival (OS) ranges from 7–12 months following chemotherapy compared with the 3–5 months that can be expected with best supportive care alone Citation[4]. In addition, improved quality of life has been reported in patients undergoing chemotherapy compared with best supportive care Citation[4].
Cisplatin and 5-fluorouracil (5-FU) have been the mainstay of treatment for metastatic gastric cancer (MGC). 5-FU is one of the most effective and widely used drugs in this tumor type, with manageable toxicity and single-agent response rates in the region of 20% Citation[5]. Improved response rates can be achieved with combination chemotherapy Citation[6], although the side effects and demanding administration schedules associated with combination chemotherapy can place a considerable physical and psychological burden on patients. Consequently, regimens have been developed that aim to minimize this burden while maintaining or improving outcomes. One popular option is to administer 5-FU by continuous infusion, thereby reducing the risk of myelotoxicity compared with bolus administration Citation[7], and allowing patients to receive their treatment on an outpatient basis. This regimen is not without drawbacks, however: ambulatory continuous infusion can impact on patients’ quality of life and central venous lines are associated with infection, bleeding, and venous thrombosis Citation[8].
More recently, oral agents have been developed in an attempt to minimize the inconvenience of chemotherapy. One such agent is UFT (tegafur–uracil), which is administered in combination with an agent such as leucovorin (folinic acid). Overall response rates of approximately 28% have been demonstrated in three phase II trials of UFT in MGC Citation[9–11]. This regimen was well tolerated, the most common toxicities being anorexia, nausea, vomiting, diarrhea, and oral mucositis.
On the basis of the mechanism of action and toxicity profile of UFT, as well as its efficacy in MGC and ease of administration, we undertook the present study to assess the antitumor activity and tolerability of the combination of cisplatin, epirubicin, UFT, and leucovorin (PELUF) in patients with MGC.
Material and methods
Patient selection
Patients were eligible for inclusion in this study if they had histologically confirmed advanced or metastatic gastric adenocarcinoma, with at least one unidimensionally measurable lesion (i.e., at least one diameter ≥2 cm, according to Response Evaluation Criteria In Solid Tumors [RECIST] guidelines Citation[12]). Previous chemotherapy for advanced or metastatic disease was not allowed. Patients who had received previous adjuvant chemotherapy were eligible if they had remained disease-free for at least six months after completion of the adjuvant therapy. Other eligibility criteria were: age ≥18 years, World Health Organization (WHO) performance status (PS) 0–2; adequate hematologic parameters (hemoglobin ≥9 g/dl, absolute neutrophil count ≥1.5×109/l, and platelets ≥100×109/l); hepatic function [total bilirubin ≤1.5 mg/dl, serum transaminases ≤3×upper normal limit or ≤5×upper normal limit in cases of hepatic metastases], and renal function [serum creatinine ≤1.5 mg/dl] were required. Patients with severe cardiac dysfunction, liver metastases involving > 50% of the liver parenchyma, chronic diarrhea, peripheral neuropathy grade > 1, previous irradiation affecting > 30% of the active bone marrow, unresolved bowel obstruction, or malabsorption syndrome, were excluded. The study protocol was approved by ethics and scientific committees of the participating centers and all patients gave written informed consent.
Treatment plan
Cisplatin 60 mg/m2 was administered intravenously (i.v.) using standard hydration on day 1, along with epirubicin 50 mg/m2. All patients received ondansetron and dexamethasone for the prevention of emesis before administration of cisplatin and epirubicin. UFT 300 mg/m2 was administered orally in conjunction with leucovorin calcium 30 mg/day in two divided daily doses for 21 days (day 1 afternoon to day 22 morning), followed by a seven-day rest period. Anti-diarrheal therapy (high-dose loperamide) was administered according to guidelines Citation[13]. Courses were repeated every four weeks until disease progression, unacceptable toxicity, or the patient refused further treatment.
Dose modification
Patients were assessed for toxicity before each cycle using the National Cancer Institute Common Toxicity Criteria (NCI-CTC version 2). UFT, epirubicin, and cisplatin doses were adjusted for hematologic adverse events as follows: for grade 1 neutropenia at day 29, no dose reductions or schedule adjustments were required; for grade 2/3 neutropenia or grade 1/2 thrombocytopenia at day 29, the treatment was delayed until the toxicity had resolved to grade 1 neutropenia and platelet count ≥100×109/l. Doses of all drugs were reduced by 25% in subsequent cycles in the event of grade 4 neutropenia, febrile neutropenia, or grade 3/4 thrombocytopenia lasting for three days or more. Febrile neutropenia was treated with i.v. broad-spectrum antibiotics and granulocyte colony-stimulating factor (Neupogen®, Roche; 5 µg/kg/day subcutaneously).
Dose-adjustment criteria for cisplatin were based on serum creatinine levels immediately prior to each cycle. For levels ≤1.5 mg/dl, the full dose of cisplatin was given; for levels in the range 1.5–2.5 mg/dl, 50% of the cisplatin dose was administered; patients with levels > 2.5 mg/dl were excluded from the study. The UFT dose was adjusted for non-hematologic adverse events as previously reported Citation[14]. Patients were required to meet all of the following criteria to begin the next cycle of treatment: neutrophil count ≥1.5×109/l; platelet count ≥100×109/l; resolution or improvement to grade 0/1 of clinically significant non-hematologic adverse events (including diarrhea and mucositis). Patients were excluded from the study if treatment was delayed for three weeks or more.
Assessment of compliance and dose intensity
Compliance with UFT therapy was monitored by questioning patients and counting their remaining pills at each outpatient visit. The ratio of the actual administered dose to the scheduled dose was calculated. Dose intensity was defined as the total amount of drug given, divided by the number of weeks on therapy.
Patient evaluation
Pretreatment evaluation, which had to be performed within two weeks before study entry, included a detailed medical history and physical examination, complete blood count (CBC) with differential and platelet count, blood chemistry, serum levels of carcinoembryonic antigen and carcinoma antigen 19/9, and computed tomography scans of the chest and abdomen. During treatment, a CBC with differential and platelet count was performed every two weeks; in cases of grade 3/4 neutropenia/thrombocytopenia, or febrile neutropenia, a CBC was performed daily until hematologic recovery. In addition, patients were clinically assessed and blood chemistry was performed on day 29 before each treatment cycle.
Response to treatment was evaluated after every two chemotherapy cycles (every eight weeks) or sooner if clinically indicated, until tumor progression. Tumor response was classified according to RECIST guidelines Citation[12]. Patients with a complete response or partial response required a confirmatory disease assessment at least four weeks later. Patients with unconfirmed tumor response were not regarded as responders.
Statistical analysis
The primary endpoint of the study was the objective response rate. Secondary endpoints were time to progression (TTP), OS, and safety. The optimal Gehan two-stage phase II design was used to determine the sample size Citation[15]. We planned to enroll at least 30 assessable patients, with a target minimum response rate of 30%. If no objective tumor response were seen among the first nine assessable patients in the study, then the probability of a response rate of ≥30% would be < 5% and the study would be discontinued. One or more responses would indicate that continuation was warranted, and approximately 30 patients would be required to estimate the 95% confidence interval (CI) for the true response rate with a maximum width of 36%. TTP was calculated from the date of study entry to the date of progressive disease; OS was measured from the date of entry to the date of last follow-up or death. TTP and OS were analyzed according to the Kaplan-Meier method and were updated to August 30, 2005.
Results
Patient characteristics
Between February 2003 and January 2005, 39 patients with MGC were entered into this multicenter trial. All patients were evaluable for efficacy and toxicity. Pre-treatment characteristics are shown in . None of the 39 patients had previously received chemotherapy for advanced disease; four patients had received adjuvant chemotherapy that did not involve cisplatin, epirubicin, or UFT before entering the study (median 15 months before study entry; range 7–27 months).
Table I. Patient characteristics.
Efficacy and survival
Two complete responses and 13 partial responses were observed in the 39 evaluable patients to give an overall response rate of 38% (95% CI 24–52%) (). Site-specific response rates were: 31% for lymph nodes; 67% for liver metastases; 45% for lung metastases; 18% for peritoneal metastases; and 11% for local disease. There was no difference in response rate according to tumor location (proximal stomach: 3 of 9 patients [33%]; gastric body: 6 of 18 patients [33%]; distal stomach: 4 of 12 patients [33%]) or tumor status (locally advanced disease: 3 of 7 patients [43%]; metastatic disease: 12 of 32 patients [38%]). Responses were seen in 1 of 4 patients (25%) who had received previous adjuvant chemotherapy and in 14 of 35 patients (40%) who were chemotherapy naïve. Responses were also detected in 12 of 31 patients (39%) whose primary tumor had not been resected and in 3 of 8 patients (38%) whose primary tumor had been resected. Median TTP was 6.5 months (95% CI 5.5–7.5 months) () and median OS was 9.5 months (95% CI 8.5–13.5 months) (), with a one-year survival rate of 36% (95% CI 21–51%). The median follow-up period was 19 months (range 17–21 months).
Figure 1. Time to progression (TTP) in patients with metastatic gastric cancer treated with the combination of cisplatin, epirubicin, tegafur–uracil, and leucovorin (PELUF). TTP was analyzed according to the Kaplan-Meier method and was updated to August 30, 2005.
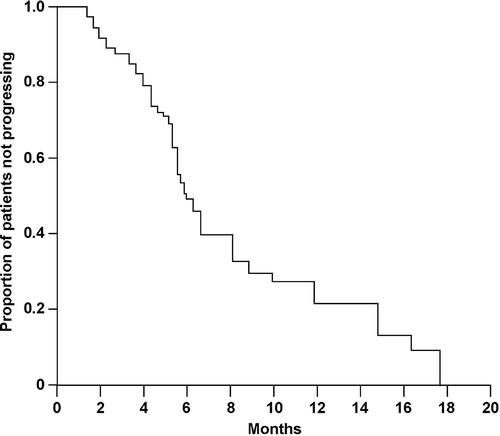
Figure 2. Overall survival (OS) in patients with metastatic gastric cancer treated with the combination of cisplatin, epirubicin, tegafur–uracil, and leucovorin (PELUF). OS was analyzed according to the Kaplan-Meier method and was updated to August 30, 2005.
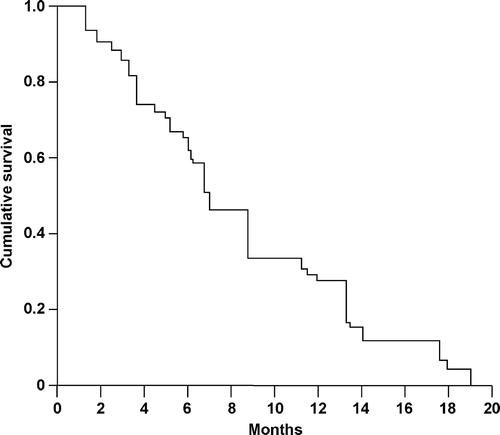
Table II. Objective response following treatment with cisplatin and epirubicin plus UFT and leucovorin.
Adverse events
Hematologic toxicity data are shown in . A total of 196 cycles were analyzed in 39 patients who received a median of five cycles (range 2–8 cycles). The most important toxicity was myelosuppression, including grade 3/4 neutropenia in eight patients (20%) during 33 cycles (17%). Two patients (5%) experienced febrile neutropenia. Non-hematologic adverse events are shown in Table IV. There were no treatment-related deaths.
Table III. Grade 3/4 toxicity following treatment with cisplatin and epirubicin plus UFT and leucovorin.
Doses were reduced in 53 cycles (27%) for the following reasons: hematologic toxicity (19%); diarrhea (4%); and nausea/vomiting (4%). Treatment was delayed in 31 cycles (16%). The median dose intensity for UFT over all treatment cycles was 3 055 mg/m2/week (range 2 088–4 118 mg/m2/week); for cisplatin this was 22.5 mg/m2/week (range 18.5–28 mg/m2/week) and for epirubicin it was 19 mg/m2/week (range 16–24 mg/m2/week); these values correspond to 85%, 90%, and 90% of the planned dose intensities, respectively.
Discussion
The results of the present study show that the combination of cisplatin, epirubicin, UFT, and leucovorin (PELUF) is an active and well-tolerated first-line therapy option for patients with MGC. An overall response rate of 38% was observed, with two complete responses and 13 partial responses. Of particular significance is the fact that 38% of patients had liver involvement at the time of study entry and the primary tumor had not been resected in 79% of patients. A 67% response rate was seen in liver metastases and a 45% response rate in lung metastases. As previously reported by Jeen et al. Citation[16], the response rate was slightly higher in patients with locally advanced diseases compared with metastatic disease (43% vs. 38%, respectively).
The median survival of ten months observed in this study is comparable with that reported by Kim et al. Citation[17], who used a similar drug combination in patients with MGC, but with a higher response rate (54%). In that study, however, patients received a higher UFT dose (360 mg/m2/d) than is generally used, and experienced a higher rate of WHO grade 3/4 toxicities compared with our study: leukopenia 38% vs. 25%; nausea/vomiting 30% vs. 8%; oral mucositis 14% vs. 5%; and diarrhea 11% vs. 8%, respectively. Consequently, we decided to use a lower dose of UFT (300 mg/m2/day) and a lower dose of leucovorin (30 mg/day) in our study in an attempt to reduce these toxicities. In their single-center study, in which the higher UFT dose was also used, Jeen et al. Citation[16] reported a somewhat higher response rate (58%) and longer survival (15 months) for patients treated with the Kim regimen. Once again, however, the rate of grade 3/4 neutropenia (42%) was higher that observed in our study.
The results of the present study are comparable with those achieved in several trials using the combination of epirubicin, cisplatin, and continuous infusion of 5-FU (ECF regimen), although inter-trial comparisons must be interpreted with caution. Response rates of 45% and 42%, and median survival times of 8.9 and 9.4 months have been reported in two randomized studies using ECF Citation[18], Citation[19]. However, complications resulting from the central venous line that is mandatory for continuous infusion of 5-FU occurred in 15% of ECF-treated patients in the study by Webb et al. Citation[18], while 16% of patients in the study conducted by Ross et al. had exit-site infections and a further 7% experienced thrombosis Citation[19]. Diarrhea, a commonly reported side effect of treatment with UFT, was generally mild to moderate in our patients as a result of the aggressive management strategy employed in the study.
Higher response rates have been reported for some of the newer-generation combination regimens than we observed for PELUF in our study. In preliminary communications, a response rate of 68% and a TTP of 7.7 months were reported for the combination of docetaxel, capecitabine, and cisplatin Citation[20], while a response rate of 68% and a median survival of 12.4 months were reported for the combination of docetaxel, carboplatin, and 5-FU Citation[21]. Both regimens were associated with considerable hematologic toxicities; grade 3/4 leukopenia and neutropenia were reported in 40% of patients treated with docetaxel, capecitabine, and cisplatin and grade 3/4 anemia occurred in 33% of patients treated with docetaxel, carboplatin, and 5-FU.
While improving response rates is an important goal in cancer chemotherapy, it is important to balance efficacy with the ability of patients to tolerate the toxicities that may be associated with some of the more aggressive combinations and to match therapies accordingly. A regimen such as PELUF, which extends survival without subjecting patients to excessive toxicities, has the potential to play an important role in the treatment of patients with advanced or metastatic gastric cancer. The PELUF regimen is a convenient treatment option, comprising outpatient administration of cisplatin and epirubicin on day 1 of the 28-day cycle, with oral administration of UFT and leucovorin for 21 days. Also reported in this issue is a phase I study into the efficacy and toxicity of EXE, a combination of oxaliplatin, oral capecitabine and epirubicin, in previously untreated patients with unresectable gastric cancer Citation[22]. The authors demonstrated good tolerability and promising efficacy, with a median overall survival of 9.2 months, and phase II studies are planned. With the continuing search for well-tolerated and convenient regimens, it is likely that oral administration of chemotherapeutic agents, which is preferred by patients over i.v. treatment providing efficacy is not compromised Citation[23], Citation[24], will play a greater role in chemotherapy in the future.
Although studies have demonstrated good compliance with oral chemotherapy regimens Citation[25], Citation[26], poor compliance can also be a concern, especially if highly emetogenic agents are used. In our study, however, compliance with UFT was not compromised by the inclusion of cisplatin in the regimen. This is likely to be a result of the routine use of anti-emetic treatment, as well as the selection of a reasonable dose intensity of the UFT component. Since patients in our study took approximately 85% of the projected UFT dose, over-compliance does not appear to be an issue with the PELUF regimen.
In conclusion, cisplatin and epirubicin plus UFT and leucovorin (PELUF) is an effective and well-tolerated first-line treatment for MGC. This combination is a good alternative to prolonged ambulatory treatment using a portable infusion pump and is thus more convenient for patients than regimens that include infusional 5-FU. The results of the present study support the role of UFT as a replacement for 5-FU in the treatment of MGC. Further evaluation of this promising combination is warranted.
Financial support for the study was provided by Bristol-Myers Squibb (Israel). The authors certify that they have not entered into any agreement that could interfere with their access to the data on the research, nor upon their ability to analyze the data independently, to prepare manuscripts, and to publish them.
References
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001; 2: 533–43
- Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000; 88: 921–32
- Greenlee, RT, Hill-Harmon, MB, Murray, T, Thun, M. Cancer statistics, 2001. CA Cancer J Clin 2001;51:15–36. Erratum in CA Cancer J Clin 2001;51:144.
- Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8: 163–8
- Findlay M, Cunningham D. Chemotherapy of carcinoma of the stomach. Cancer Treat Rev 1993; 19: 29–44
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005; 10(Suppl 3)49–58
- Lokich JJ, Ahlgren JD, Gullo JJ, Phillips JA, Fryer JG. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program Study. J Clin Oncol 1989; 7: 425–32
- Poorter RL, Lauw FN, Bemelman WA, Bakker PJ, Taat CW, Veenhof CH. Complications of an implantable venous access device (Port-a-Cath) during intermittent continuous infusion of chemotherapy. Eur J Cancer 1996; 32A: 2262–6
- Ota K, Taguchi T, Kimura K. Report on nationwide pooled data and cohort investigation in UFT phase II study. Cancer Chemother Pharmacol 1988; 22: 333–8
- Kim YH, Cheong SK, Lee JD, Park JS, Shin SW, Kim JS. Phase II trial of oral UFT and leucovorin in advanced gastric carcinoma. Am J Clin Oncol 1996; 19: 212–6
- Ravaud A, Borner M, Schellens JH, et al. UFT and oral calcium folinate as first-line chemotherapy for metastatic gastric cancer. Oncology (Williston Park) 1999; 13(7 Suppl 3): 61–3
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16
- Wadler S, Benson AB, Engelkin C, et al. Recommended guidelines for treatment of chemotherapy-induced diarrhea. J Clin Oncol 1998; 16: 3169–78
- Woo IS, Moon do H, Shim BY, et al. A phase II study of epirubicin, cisplatin and uracil-tegafur for advanced gastric carcinoma. Jpn J Clin Oncol 2005; 35: 13–7
- Gehan EA. The determination of the number of patients required in a preliminary and follow-up trial of a new chemotherapeutic agent. J Chronic Dis 1961; 13: 346–53
- Jeen YT, Yoon SY, Shin SW, et al. Phase II trial of epirubicin, cisplatin, oral uracil and tegafur, and leucovorin in patients with advanced gastric carcinoma. Cancer 2001; 91: 2288–93
- Kim YH, Kim BS, Seo JH, et al. Epirubicin, cisplatin, oral UFT, and calcium folinate in advanced gastric carcinoma. Oncology (Williston Park) 1999; 13(7 Suppl. 3): 64–8
- Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15: 261–7
- Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002; 20: 1996–2004
- Kang, Y-K, Kim, T-W, Chang, H-M, et al. A phase I/II trial of docetaxel, capecitabine, and cisplatin as a first line chemotherapy for advanced gastric cancer. Proc Am Soc Clin Oncol 2004;22:329s, (Abstract No. 4066).
- Elsaid, AA, Elkerm, Y. Final results of a randomized phase III trial of docetaxel, carboplatin and 5FU versus epirubicin, cisplatin and 5FU for locally advanced gastric cancer. Proc Am Soc Clin Oncol 2005;23:311s, (Abstract No. 4014).
- Dupont, J, Jensen, HA, Jensen, BV, Pfeiffer, P. Phase I study of short-time oxaliplatin, capecitabine and epirubicin (EXE) as first-line therapy in patients with non-resectable gastric cancer. Acta Oncol, (in press).
- Borner MM, Schoffski P, de Wit R, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. Eur J Cancer 2002; 38: 349–58
- Twelves C, Gollins S, Grieve R, Samuel L. A randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Ann Oncol 2006; 17: 239–45
- Lee CR, Nicholson PW, Souhami RL, Slevin ML, Hall MR, Deshmukh AA. Patient compliance with prolonged low-dose oral etoposide for small cell lung cancer. Br J Cancer 1993; 67: 630–4
- Lee CR, Nicholson PW, Ledermann JA, Rustin GJ. Patient compliance with prolonged oral altretamine treatment in relapsed ovarian cancer. Eur J Gynaecol Oncol 1996; 17: 99–103
