Abstract
Chemotherapy dosing only based on body surface area (BSA) results in marked pharmacokinetic and toxicity variations, which may result in an inferior outcome for some patients. A toxicity-based dosing schedule for individually tailored treatment with granulocyte colony-stimulating factor (G-CSF) supported 5-fluorouracil (F), epirubicin (E) and cyclophosphamide (C) (dFEC) was developed and studied in patients with metastatic breast cancer with the purpose to determine its efficiency and toxicity. Twenty-six women, median age 48 years, were included and the individual E and C doses were tailored stepwise based on the recorded hematological toxicity. Twenty-one patients (81%; 95% confidence interval (CI), 66% to 96%) had an objective response, including six complete responses (23%; CI, 7%–39%). At median follow-up of 113 months, the median time to progression and median overall survival were 14 and 36 months, respectively. The delivered dose intensity was high but varied substantially between patients (ranges F 126–202, E 14.4–36.0, C 160–510 mg/m2/w). The dominating grade III/IV toxicity was nausea (12% of patients) and febrile neutropenia (31% of patients). The tailored and dose-escalated FEC was highly active and feasible in metastatic breast cancer and may provide a pragmatic way of overcoming the shortcomings of standard BSA-based dosing.
The importance of chemotherapy dose-intensity in advanced as well as early breast cancer has been debated for decades. Several randomized studies Citation[1–3] have demonstrated that doses lower than conventional usually lead to inferior response rates and even compromised survival. The meta-analysis by Fossati et al. also demonstrated a statistically significant survival benefit from high chemotherapy doses within the conventional range Citation[4]. The benefit of doses higher than standard, however, is still controversial. Several studies on high-dose chemotherapy with stem cell rescue have not shown superior results by this procedure compared with conventional doses Citation[5], Citation[6]. Thus, it seems reasonable to avoid very low as well as very high doses when using chemotherapy in the treatment of breast cancer.
A major problem in this effort is the marked inter-individual variability in the pharmacokinetics (Pk) and pharmacodynamics (Pd) of cytotoxic drugs. Dosing according to patients' body surface area (BSA) is clearly not a solution to this variability Citation[7]. In one study patients were treated with single drug epirubicin in doses from 45 to 135 mg/m2 in prospectively allocated cohorts Citation[8]. Some patients treated at the lowest dose level actually experienced similar hematological toxicity as others treated at the highest dose levels. This means that moderate dose escalation may still leave some patients far below their tolerance levels, while others are at their tolerance limit already at ordinary doses. An important annotation in this subject is that retrospective data has showed that patients who experience pronounced myelosuppression during adjuvant treatment have improved survival compared with patients with less myelosuppression Citation[9–12].
In the present study we attempted to overcome the problem with the combination of inter-individual differences in Pk and Pd by escalating doses according to each patient's bone marrow tolerance. In order to further increase the dose intensity, granulocyte colony-stimulating factor (G-CSF) was added. This tailor made and dose-escalated combination of 5-fluorouracil, epirubicin and cyclophosphamide has been compared with standard FEC followed by stem cell supported high-dose chemotherapy, when used in the adjuvant setting Citation[13]. In this phase II trail we used the toxicity-based dosing strategy in the treatment of metastatic breast cancer to explore the efficiency and feasibility.
Material and methods
Patient selection
Patients were required to have histologically confirmed breast cancer with measurable or evaluable metastatic disease. Patients with bone metastases only were included if lytic lesions were present. Other requirements included: (a) age < 60 years; (b) expected survival > 12 weeks; (c) no prior chemotherapy for metastases; (d) no prior adjuvant anthracyclines; (e) no symptoms of heart disease; (f) no signs of brain metastases; (g) no signs of severe organ dysfunction (i.e. bilirubin < 42 µmol/L, creatinine < 150 µmol/L, haemoglobin > 100 g/L, white blood cell count (WBC) > 3.5×109/L, and platelet count > 100×109/L); (h) no concomitant hormonal therapy except for vaginal oestrogen; (i) no radiotherapy < 6 months towards a sole measurable lesion; (j) no prior invasive malignancies with the exception of radically treated carcinoma-in-situ of the cervix or basalioma of the skin. All patients received oral and written information and provided informed consent. The ethical committees at both centers and the Swedish Medical Products agency approved of the study.
Investigations before treatment start
Before start of treatment all patients underwent ECG, pulmonary x-ray, bone scan, laboratory investigations and clinical examination. Pathological uptakes on bone scan were investigated by x-ray. Ultrasonography or computed tomography of the liver was performed if warranted by signs and symptoms or if one or more liver enzymes (AST, ALT, LDH, alkaline phosphatase or bilirubin) were increased.
Treatment
The patients initially received dFEC at dose level 1 (), consisting of cyclophosphamide (900 mg/m2) given as a 15 min i.v. infusion followed by 5-fluorouracil (600 mg/m2) i.v. bolus injection, and finally, epirubicin (75 mg/m2) given as a 1 h i.v. infusion. G-CSF (lenograstim) was administered subcutaneously (0.263 mg) daily beginning on day 3 of chemotherapy and continuing until day 12, or until leukocytes had recovered to > 1.0×109/L. Ciprofloxacin 500 mg bid orally was given profylactically day 3–12. Blood samples were taken day 8, 11/12, 15, and 22 (planned day for next cycle) for WBC (measuring both polynuclear and mononuclear leukocytes), platelet count and hemoglobin level.
Table I. Dose levels used for stepwise escalation or reduction
The dose-modification schedule () was based on WBC nadir and platelet counts. The target WBC nadir was < 1.0×109/L for maximum four days and platelet nadir between 25 and 75, otherwise the next course was escalated or reduced according to a dose-level protocol (). For dose-level 2 and above, mesna at 20% of the cyclophosphamide dose was given i.v. or orally in double dose at 0, 4 and 8 h after the cyclophosphamide infusion.
Table II. Dose-modification schedule based on the recorded myelotoxicity after previous treatment cycle
Nine courses were to be given. In the case of complete response (CR) consolidation with high-dose treatment with autologous bone marrow transplantation could be considered. For patients with partial response (PR) or stable disease (SD) continuing standard FEC q 5w were proposed until a cumulative epirubicin dose of 1 000 mg/m2 was reached. In the case of progressive disease (PD), dFEC was stopped. Other reasons for discontinuing were prohibitive toxicity, or the patient's or physician's discretion to stop.
Evaluation
Tumor evaluation was performed after every third cycle and the response to treatment was evaluated according to WHO criteria Citation[14]. The same method was used as in pretreatment investigations. Response in bone metastases was based on plain x-ray investigations of affected bone. If the disease had not progressed before completion of the dFEC treatment, repeated investigations were recommended at every third cycle when on chemotherapy, or on a three monthly basis. All images were reviewed by an experienced radiologist (GÅ) after completion of the study.
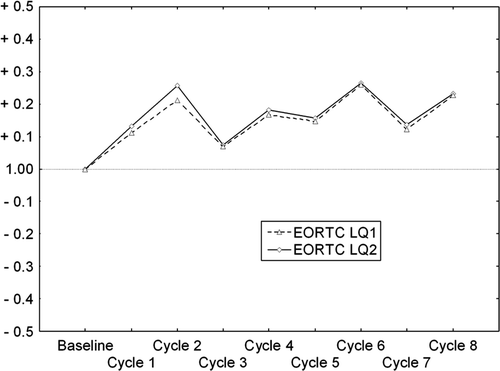
Toxicity was reported in accordance with NCI common toxicity criteria. All patients self-assessed their quality of life (QoL) before treatment starts and during all cycles, except cycle number nine. The third generation EORTC QLQ-C30(+3) questionnaire with the breast cancer module BR23, was used on day 1 before next cycle. Two different endpoints (LQ1 and LQ2) were representing the global QoL according to the EORTC QLQ-C30 scoring manual Citation[15], and are presented separately here. Only the data of the two global QoL endpoints are reported in this paper.
Objectives and statistical considerations
The primary objective of this phase II study was to determine the objective response rate. Secondary objectives were overall survival (OS), time to progression (TTP), dose intensity, toxicity and QoL. A number of at least twenty evaluable subjects were to be included. The survival distributions for time to progression and overall survival were estimated using the Kaplan-Meier method. Time to progression was measured from the date of study entry until the date of progression or relapse. The Pearson product-moment method was used for correlations and the Student t-test for comparisons between groups. The Wilcoxon matched pairs test was used for analysis of the QoL.
Results
Twenty-six women were enrolled onto the study between September 1994 and January 1997. Twenty-two patients were included at Uppsala university hospital and four patients at the center in Örebro university hospital, which opened for inclusion in May 1996. All eligible patients at the two centres were considered for the study and received oral and written information. Pretreatment characteristics are shown in . The patients had newly diagnosed metastatic breast cancer except for two patients who had received endocrine treatment for metastatic disease during 30 and two months respectively. Seven patients received consolidating high-dose chemotherapy with ABMT after completion of the study treatment. The median follow-up time was 113 months.
Table III. Patient characteristics
Delivered dose and dose intensity
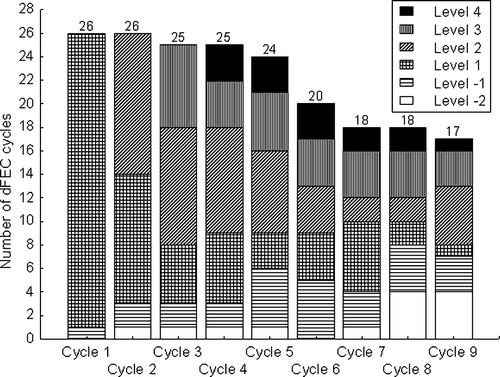
A total of 199 cycles of dFEC were administered and the median number of cycles was nine. The distribution of the dose levels is shown in . The given average dose intensity was F 185 mg/m2/w, E 26.4 mg/m2/w and C 336 mg/m2/w (representing 93%, 132% and 168% of intended doses of standard FEC). The individual dose intensity varied considerably with F ranging from 127 to 201 mg/m2/w (63%–101% of standard FEC), E ranging between 14.4 and 36.0 mg/m2/w (72%–180%) and C dose intensity ranging from 159 to 510 mg/m2/w (80%–255%). The maximum tolerated dose ranged from level -1 for two patients up to level 4 for five individuals. There was a weak negative correlation between the dose intensity of E (r = − 0.35) and C (r = − 0.33) and age, however it was not significant (p = 0.08 and 0.10, respectively). The presence of liver (9 patients) or bone (13 patients) metastases did not influence the delivered dose intensity. Nor did previous adjuvant radiotherapy, endocrine therapy or chemotherapy correlate to the delivered dose intensity.
Figure 1. Stacked column diagram showing the distribution of different dose levels during the study treatment of 9 cycles. The number of given dFEC cycles (n = 199) is presented at the Y-axis at each cycle (X-axis). The highest dose levels were delivered at cycle 4-6 and all patients but one (wrongly treated with level -1 at the first cycle), received the level 1 at the start.
Response and Survival
The response rate is shown in . The overall response rate was 81% (CI 66%–96%). Median TTP was 14 months and median OS was 36 months (). Among 13 patients with visceral metastases, ten responded (77%) with CR in three patients (23%). Median TTP for this subgroup was 11 months, with a median OS of 22 months. At 113 months, two patients are still alive. Both were initially in CR and received double high-dose therapy with ABMT but have now relapsed in their breast cancer disease.
Figure 2. Kaplan-Meier diagram showing time to progression (TTP) and overall survival (OS) for patients in the present study (n = 26).
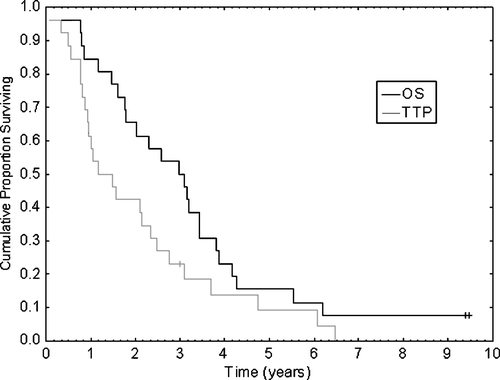
Table IV. Treatment responses
For the 13 patients receiving the highest dose intensity, 11 responded compared with ten patients among the 13 patients with the lowest dose intensity. There was no significant correlation between E and C dose intensity and OS or TTP.
Adverse events and Quality of Life
The toxicity per cycle and per patient by worst grade experienced during the treatment is listed in . Due to the design of the treatment schedule, all patients experienced grade IV leucopenia. Fever was common (22% of the cycles and in 69% of the patients), but mostly not associated with leucopenia or neutropenia. Ten episodes of fever above 38.0°C related to a period of leucopenia or neutropenia grade IV occurred in eight patients. They were hospitalized and treated with i.v. antibiotics. No septic death occurred.
Table V. Worst grade reported toxicity per cycle (n = 199) and per patient (n = 26)
There was no sign of non-hematological grade IV toxicity. The most common grade III toxicity was nausea/ vomiting (12% of patients). No significant cardiac toxicity was seen except in one patient, who 4.5 years after study completion showed moderate to severe symptoms of cardiac heart failure (CHF), responsive to therapy. She had also received two stem-cell supported high-dose treatments with CTCb and paclitaxel respectively, as consolidation therapy after completion of the study. One patient developed acute myeloid leukemia type M1, 27 months after completion of the study treatment and died five months later. Also this patient had previously received high-dose treatment with CTCb after completion of the study and had no signs of breast cancer relapse. Other reported possible related or non-related adverse events causing hospitalizations during the study period were thrombosis (2 patients), diarrhea (1 patient), gastritis (1 patient), gastro-enteritis (1 patient) and tumor related pain (3 patients).
QoL data were obtained from 22 patients. There was a slight increase in global QoL compared with baseline () for both global endpoints of the EORTC questionnaire indicating better QoL during treatment. Individual data from the EORTC instrument, comparing baseline and each patients last cycle, showed, however, no difference (p = 0.14 and p = 0.26, Wilcoxon matched pairs test).
Discussion
Delivery of standard doses of chemotherapy based on patients’ BSA will result in marked inter-individual variations in toxicity Citation[7], Citation[16], which may be explained by the large differences in drug clearance and sensitivity between patients Citation[17], Citation[18]. Retrospective studies in early breast cancer have indicated that lack of toxicity could be a sign of under-treatment which may compromise survival Citation[9–12].
In the present study we attempted to overcome the problem with inter-individual Pk and Pd variability by escalating doses to pronounced but equivalent bone marrow toxicity. To achieve as high dose intensity as possible, G-CSF was added day 3–12. The feasibility of this approach with leukocyte nadir based dosing has previously been reported in the adjuvant setting where tailored FEC was followed by fewer breast cancer relapses compared with standard FEC followed by marrow supported high-dose therapy Citation[13], Citation[19]. Due to the application of an individual dose escalation schedule in both the adjuvant trial and the present study, the achieved dose intensities in these two studies are likely to be very close to the maximum tolerance for FEC q 3 weeks supported with G-CSF. However, the achieved dose intensity was slightly higher in the previously reported adjuvant trial compared with this study on women with advanced breast cancer. The adjuvant trial reported median weekly E dose was 33 mg/m2/w, compared with an average dose of 26.4 mg/m2/w in this study. Corresponding dose intensity for C was 431 versus 336 mg/m2/w in the early and metastatic breast cancer setting, respectively. This finding could indicate that presence of metastases may have negatively influence the dose tolerance level. We did not, however, find any correlations between tolerated dose and presence of bone or liver metastases.
Higher doses of anthracyclines will produce higher response rates and may prolong survival Citation[4], Citation[20]. The response rate observed in this study (81%) is impressive, but does not differ from earlier phase II results from G-CSF-supported intensified anthracycline based treatments in which the dosing was based on BSA with response rates in the range 75–89% Citation[21–24].
One reason for the high response rate may be the fact that rather few patients (46%) had metastasis to two or more organs. The reason is probable that the treatment option was recommended to all patients up-front when metastatic disease was diagnosed to hopefully achieve a CR followed by high-dose dose treatment with ABMT. Today many receptor positive patients would be treated with endocrine treatment up-front, and only later, in a more severe metastatic situation, would be recommended chemotherapy.
Interestingly, the response in present study was unrelated to given dose intensity, a finding also noticed in the adjuvant study Citation[13]. This observation could verify the hypothesis that toxicity-based dosage to similar hematological toxicity avoids under-dosing and is equal efficient independent to achieved individual dose intensity.
In the management of metastatic breast cancer, a major objection against the use of high dose intensity is the increased incidence of severe acute toxicity. At time of the design of the study high-dose therapy with ABMT was considered a promising option to achieve long-time DFS or possible cure for the subgroup of metastatic patients who had CR after induction chemotherapy. Therefore, we aimed at grade IV leucopenia, but for a maximum of four days which we thought to be reasonably safe. Today, when the concept of high-dose chemotherapy is questioned, we would rather aim for grade III leucopenia when using toxicity-based dosing in the palliative situation. However, in the study the non-hematological toxicity was moderate and no septic deaths occurred. The reported QoL did not decrease during the treatment (). Nevertheless, one third of the patients (8/26), required hospitalization at one or more times during the study because of febrile leucopenia grade III.
A major complication in the Scandinavian adjuvant study was the high incidence (3.6%, 10/274) of acute leukemia or myelodysplastic syndrome in the dFEC-arm Citation[13]. In the present study, G-CSF was used less intensively (10 days instead of 15) and started one day later (day 3) than in the SBG 9401 study. Other differences to the SBG 9401 study are the use of lenograstim instead of filgrastim and the use of a fixed dose of G-CSF (0.263 mg per day). Nevertheless, one heavily treated patient developed acute myeloid leukemia. However, the rather late occurrence of this complication together with the fact that this patient also had received marrow-supported high-dose therapy makes the conclusion on cause less obvious.
Another potential long-term side effect of anthracycline therapy is the cardiotoxicity. Only one patient developed symptomatic CHF in our cohort despite a median cumulative epirubicin dose of 640 mg/m2, range 134 mg/m2 to 1 244 mg/m2.
The leukocyte nadir based dosing has the disadvantage of the extra blood sampling around the predicted days for nadir, but is otherwise as easy to administrate as BSA-based dosing. Another disadvantage of tailoring treatment based on Pd effects (i.e. toxicity) is the risk for under-treatment during cycle one to three due to the relatively slow escalation, e.g. patients tolerating level 4 were step-wise escalated until cycle 4 (). New schedules, allowing faster escalation for patients with initial low toxicity are now under investigation.
We have here studied individually tailored dosing of FEC, but the concept may be used for other regimens or drugs there a large variability in tolerance is seen and myelotoxicity is dose limiting i.e. docetaxel.
In summary the present tailored toxicity-based regimen showed encouraging activity with normalized myelotoxicity between patients despite large differences in dose intensity. The individually tailored dosing principle may provide an alternative to the BSA-based dosing algorithm in the treatment of metastatic breast cancer as well as in the adjuvant setting.
The study was supported by grants from Pharmacia & Upjohn and by the Swedish Cancer Society and monitored by Rhone-Poulenc Rorer (presently Sanofi-Aventis).
References
- Carmo-Pereira J, Costa F, Henriques E, Godinho F, Cantinho-Lopes M, Sales-Luis A, et al. A comparison of two doses of adriamycin in the primary chemotherapy of disseminated breast carcinoma. Br J Cancer 1987; 56: 471–3
- French-epirubicin-study-group. Epirubicin-based chemotherapy in metastatic breast cancer patients: Role of dose-intensity and duration of treatment. J Clin Oncol 2000;18:3115–24.
- Tannock IF, Boyd NF, DeBoer G, Erlichman C, Fine S, Larocque G, et al. A randomized trial of two dose levels of cyclophosphamide, methotrexate, and fluorouracil chemotherapy for patients with metastatic breast cancer. J Clin Oncol 1988; 6: 1377–87
- Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol 1998; 16: 3439–60
- Rodenhuis S, Bontenbal M, Beex LV, Wagstaff J, Richel DJ, Nooij MA, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med 2003; 349: 7–16
- Stadtmauer EA, O'Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. Philadelphia Bone Marrow Transplant Group. N Engl J Med 2000; 342: 1069–76
- Gurney H. How to calculate the dose of chemotherapy. Br J Cancer 2002; 86: 1297–302
- Jakobsen P, Bastholt L, Dalmark M, Pfeiffer P, Petersen D, Gjedde SB, et al. A randomized study of epirubicin at four different dose levels in advanced breast cancer. Feasibility of myelotoxicity prediction through single blood-sample measurement. Cancer Chemother Pharmacol 1991; 28: 465–9
- Cameron DA, Massie C, Kerr G, Leonard RC. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer 2003; 89: 1837–42
- Poikonen P, Saarto T, Lundin J, Joensuu H, Blomqvist C. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer 1999; 80: 1763–6
- Saarto T, Blomqvist C, Rissanen P, Auvinen A, Elomaa I. Haematological toxicity: A marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br J Cancer 1997; 75: 301–5
- Mayers C, Panzarella T, Tannock IF. Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer 2001; 91: 2246–57
- Bergh J, Wiklund T, Erikstein B, Lidbrink E, Lindman H, Malmstrom P, et al. Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: A randomised trial. Scandinavian Breast Group 9401 study. Lancet 2000; 356(9239)1384–91
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Fayers PM, Hopwood P, Harvey A, Girling DJ, Machin D, Stephens R. Quality of life assessment in clinical trials--guidelines and a checklist for protocol writers: The U.K. Medical Research Council experience. MRC Cancer Trials Office. Eur J Cancer 1997; 33: 20–8
- Jakobsen P, Steiness E, Bastholt L, Dalmark M, Lorenzen A, Petersen D, et al. Multiple-dose pharmacokinetics of epirubicin at four different dose levels: Studies in patients with metastatic breast cancer. Cancer Chemother Pharmacol 1991; 28: 63–8
- Sandstrom M, Freijs A, Larsson R, Nygren P, Fjallskog ML, Bergh J, et al. Lack of relationship between systemic exposure for the component drug of the fluorouracil, epirubicin, and 4-hydroxycyclophosphamide regimen in breast cancer patients. J Clin Oncol 1996; 14: 1581–8
- Sandstrom M, Lindman H, Nygren P, Johansson M, Bergh J, Karlsson MO. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol 2006; 58: 143–56
- Bergh J, Wiklund T, Erikstein B, Fornander T, Bengtsson N-O, Malmström P, et al. Dosage of adjuvant G-CSF (filgrastim)-supported FEC polychemotherapy based on equivalent haematological toxicity to high-risk breast cancer patients. Ann Oncol 1998; 9: 403–11
- Bruzzi P, Del Mastro L, Sormani MP, Bastholt L, Danova M, Focan C, et al. Objective response to chemotherapy as a potential surrogate end point of survival in metastatic breast cancer patients. J Clin Oncol 2005; 23: 5117–25
- Bronchud MH, Howell A, Crowther D, Hopwood P, Souza L, Dexter TM. The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br J Cancer 1989; 60: 121–5
- Ferguson JE, Dodwell DJ, Seymour AM, Richards MA, Howell A. High dose, dose-intensive chemotherapy with doxorubicin and cyclophosphamide for the treatment of advanced breast cancer. Br J Cancer 1993; 67: 825–9
- Piccart MJ, Bruning P, Wildiers J, Awada A, Schornagel JH, Thomas J, et al. An EORTC pilot study of filgrastim (recombinant human granulocyte colony stimulating factor) as support to a high dose-intensive epiadriamycin-cyclophosphamide regimen in chemotherapy-naive patients with locally advanced or metastatic breast cancer. Ann Oncol 1995; 6: 673–7
- Van Hoef ME, Baumann I, Lange C, Luft T, de Wynter EA, Ranson M, et al. Dose-escalating induction chemotherapy supported by lenograstim preceding high-dose consolidation chemotherapy for advanced breast cancer. Selection of the most acceptable regimen to induce maximal tumor response and investigation of the optimal time to collect peripheral blood progenitor cells for haematological rescue after high-dose consolidation chemotherapy. Ann Oncol 1994; 5: 217–24