Abstract
A study of the possible difference in outcome for positive margins for invasive carcinoma (IC) versus ductal carcinoma in situ (DCIS), and with regard to different age categories in a large prospective cohort of patients with invasive breast cancer. A total of 2 291 BCT were analyzed. Margins were positive for IC in 8.7% and for DCIS in 4.6%. The median follow-up was 83 months. The 10-year local recurrence-free survival for negative margins vs. positive margins for IC vs. positive for DCIS for women ≤40 years were 84.4% vs. 34.6% (HR 4.5) vs. 67.5%, and for women >40 years 94.7% vs. 92.6% vs. 82.6% (HR4.2). The 10-year distant disease-free survival for negative margins vs. positive margins for IC vs. positive for DCIS women ≤40 years were 72.0% vs. 39.7% (HR 3.4) vs. 77.8%. The disease-specific survival showed a significant relation to positive margins for IC in young women. The effect of positive margin for IC seems to be limited to young women only, and is not only restricted to local control, but also to distant metastasis and survival. On the other hand a positive margin for DCIS is a risk factor for local control in women >40 years.
Positive resection margins in breast-conserving treatment (BCT) for breast cancer patients are often an indication for a re-excision or even an ablation. In recent phase III studies and newly designed studies negative margins are a prerequisite for inclusion. The general opinion is that a positive margin is associated with a worse prognosis with regard to local control Citation[1–8].
No phase III studies have been or will be designed to look at the problem of a positive margin. Sub analyses from phase III studies and retrospective studies have addressed the issue of positive margins. Margins of patients with invasive breast cancer can be positive for invasive carcinoma (IC) as well as for ductal carcinoma in situ (DCIS) or both. The outcome is diverse and in many studies no difference is being made whether the margin is positive for IC or DCIS. Neither is a distinction made in terms of patient's categories.
The aim of this study is to look in a large prospective cohort of patients with invasive breast cancer at the possible difference in outcome for positive margins for IC and DCIS, and with regard to age category.
Patients and methods
This prospective cohort of patients was started in 1983 when BCT was introduced in our region. All patients in the Twente-Achterhoek region with invasive breast cancer and treated with BCT received their radiotherapy at the Radiotherapy Department of the Medisch Spectrum Twente at Enschede. From 1983 through 2002, a total of 2 335 BCT were registered in 2 267 patients with invasive breast cancer. Pathological examination for all BCT was done in the Pathology Laboratory Oost Nederland. All patient data, including demographics, histology, staging information, treatment, and outcome were recorded and are updated regularly. Patients were classified according to the TNM-classification, 4th edition 1997. For the purposes of this study, the cut-off for analysis was December 2005.
Family history (FH) was recorded according to first-degree relative (FDR).
We defined synchronous bilateral breast cancer (BBC) as cancer diagnosed in both breasts simultaneously or within a period of three months of diagnosis of the first tumour. Metachronous contra lateral breast cancer (CBC) was defined as breast cancer occurring in the contra lateral breast more than three months after the diagnosis of the tumour in the first breast affected.
Although the grade of differentiation was recorded when known, it was not routinely reported along with the histology during the early years; and there were too few patients with known grade (51.5%) for this particular factor to be analyzed.
Margin status
Involvement of the margins of the lumpectomy specimen was defined as the presence of microscopic involvement of IC or DCIS in the inked margin. Close margins were recorded as negative. Massive involvement with IC or DCIS of the margins, defined as diffuse or multiple microscopic foci, was regarded as an indication for a re-excision. In case of focal microscopic involvement of the margin was present the surgeon was advised not to do a re-excision. The policy in our department was that minimal microscopic disease could be treated with radiotherapy. A total of 152 re-excisions were performed. Negative margins were defined as having no microscopic involvement of IC or DCIS in the inked margin of the lumpectomy specimen or after re-excision. The presence of DCIS in the lumpectomy specimen, independently of involvement of the margin with DCIS, was recorded separately. The extensive intra ductal carcinoma component was not recorded separately.Of the 2 267 patients with 2 335 BCT the margins were unknown in 3 BCT and 41 BCT were positive for both IC and DCIS. To get a clear view on the impact of IC versus DCIS at the margin, and also because of the small number, BCT with margins positive for both were excluded, leaving 2 291 BCT in 2 223 patients with invasive breast cancer for analysis.
Treatment
BCT consisted of lumpectomy with axillary clearance of levels I–III, followed by radiotherapy to the whole breast with a boost to the primary tumour area. The radiotherapy consisted in 50 Gy to the whole breast, followed by a boost of 14 Gy to the primary tumour bed. The boost dose given was the same in all patients, regardless of margin status. The localisation of the boost was determined by the location of the scar, the primary tumour location, the mammography, and if possible by placed radiopaque clips.
Adjuvant therapy consisted of radiotherapy to the regional lymph nodes or to the internal mammary chain only, and hormonal and/or chemotherapy. Regional radiotherapy, which included the axilla and the supraclavicular, and internal mammary chains, was indicated for patients with four or more positive lymph nodes and/or extra nodal disease (EN). Radiotherapy of the internal mammary chain only was indicated for those with fewer than four positive lymph nodes and no EN. In the case of medial implantation of the breast, the use of a separate anterior field for irradiation of the internal mammary chain was omitted to permit optimal irradiation of the breast.
In the late eighties adjuvant systemic therapy was introduced for patients with positive nodes. From 1992 on, all premenopausal patients with positive lymph nodes have received chemotherapy. For postmenopausal patients adjuvant hormonal therapy was given when positive lymph nodes were present. Since 1999 indications for adjuvant systemic therapy have depended not only on the lymph node status, but also in the case of a negative lymph node status on the mitotic activity index, histological grade and tumour size Citation[21]. Premenopausal women received chemotherapy and endocrine therapy when the oestrogen receptor status was positive. Postmenopausal women received primarily endocrine therapy and were offered chemotherapy when their age was <70 years. When the hormone receptor status was negative, patients were offered chemotherapy.
Statistical methods
Time to recurrence and length of follow-up were calculated from the start of BCT. To test between-group differences for categorical data χ2 tests were used, and these analyses with regard to local recurrences were performed in relation to the number of BCT. For all survival analyses, patients were censored if they had not experienced an event (local recurrence, distant metastasis) at the time of analysis or if they were lost to follow-up or were dead at the time of analysis. The local recurrence-free survival (LRFS) is defined as survival without local recurrence. An event was defined as the occurrence of a local recurrence in the treated breast.
Statistics for distant metastasis and survival were performed in relation to the number of patients and calculated by the method of Kaplan and Meier. The disease-specific survival (DSS), corrected for intercurrent death, was also calculated in relation to the number of patients. This means that for this particular analysis, data on patients who died of other causes than breast cancer were regarded as censored data. An event was defined as death due to breast cancer. The distant disease-free survival (DDFS) is defined as survival without distant metastasis in patients. An event was defined as the occurrence of a distant metastasis in the patient.
For comparison of survival distributions the log-rank test was used. Variables that were univariately related to the outcomes of interest (p < 0.10) were entered in the multivariate analyses.
The Cox proportional hazards model was used to test for the independent effect of margins status after adjusting for known prognostic factors and hazard ratios (HR) estimated with 95% confidence limits are presented. All analyses were performed using STATA Citation[9].
Results
Of the 2 291 BCT in 2 223 breast cancer patients the margins were positive for IC in 8.6% (198/2291), and in 4.6% for DCIS (108/2291). The length of follow-up ranged from 3 to 265 months, with a median of 83 and a mean of 93 months.
Characteristics of the three groups, negative margins and positive margins for either IC or DCIS are presented in . Patients with a positive margin for DCIS showed more positive lymph nodes, more lateral localisation, and received more adjuvant therapy, while patients with a positive margin for IC more often have larger tumours, and lobular carcinoma. Lymph-angioinvasion was more associated with positive margins for IC and DCIS.
Table I. Distribution of the different clinical, pathohistological, and treatment characteristics for the 2 291 breast-conserving treatments in 2 223 breast cancer patients, according to margin status for invasive carcinoma (IC) and ductal carcinoma in situ (DCIS).
The analyses showed a significant statistical interaction between margin status for IC and age. Therefore we performed the analyses with regard to outcome for women ≤40 years (165 BCT) and >40 years (2 126 BCT) separately.
Local recurrence
≤ 40 years
Of the 165 BCT margins were positive for IC in 8.4% (14/165) and in 6% for DCIS (10/165). The length of follow-up ranged from 9 to 234 months, with a median of 87 and a mean of 98 months. The 10-year LRFS rates of negative margins vs. positive margins for IC vs. positive margin for DCIS were 84.4% vs. 34.6% (HR4.5; 95% CI 1.5–13.8; p = 0.008) vs. 67.5% (HR 2.1; 95% CI 0.5–9.3; p = 0.316) ().
Figure 1. The local relapse-free survival in 165 breast-conserving treatments according to margin status for women ≤40 years.
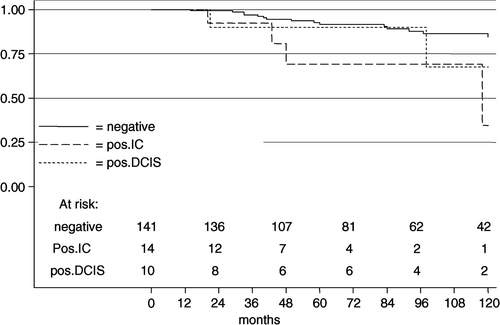
In multivariate Cox regression analysis including the only significant variable of the univariate analysis, histology and margin status, margin involvement for IC was highly significant (HR 4.6; 95% CI 1.4–15.1; p = 0.012). Margin involvement for DCIS showed no significance (HR 2.0; 95% CI 0.5–8.9; p = 0.348).
>40 years
Of the 2 126 BCT margins were positive for IC in 8.6% (184/2126), and in 4.6% for DCIS (98/2126). The length of follow-up ranged from 3 to 265 months, with a median of 83 and a mean of 93 months. The 10-year LRFS rates of negative margins vs. positive margins for IC vs. positive margin for DCIS were 94.7% vs. 92.6% (HR1.6; 95% CI 0.8–3.2; p = 0.192) vs. 82.6% (HR 4.2; 95% CI 2.2–7.9; p < 0.001) ().
Figure 2. The local relapse-free survival in 2 126 breast-conserving treatments according to margin status for women >40 years.
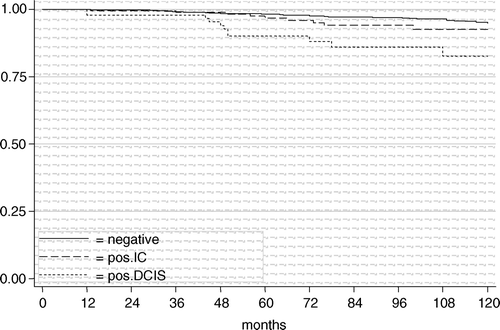
In multivariate Cox regression analysis including the significant variables of the univariate analysis (lymph-angioinvasion, presence of in situ carcinoma, and margin status) margin involvement for DCIS was highly significant (HR 3.5; 95% CI 1.6–7.7; p = 0.002), whereas margin involvement for IC was not (HR 1.4; 95% CI 0.7–2.8; p = 0.393).
Distant metastasis
≤ 40 years
The 10-year DDFS rates for negative margins vs. positive margins for IC vs. positive margin for DCIS were 72% vs. 39.7% (HR 3.4; 95% CI 1.6–7.3; p = 0.002) vs. 77.8% (HR 0.8; 95% CI 0.2–3.2; p = 0.711).
In multivariate Cox regression analysis including the significant variables of the univariate analysis, lymph-angioinvasion, BBC, and margin status, margin involvement for IC was significant (HR 2.4; 95% CI 1.0–5.6; p = 0.050). Margin involvement for DCIS showed no significance (HR 0.6; 95% CI 0.1–2.4; p = 0.435).
>40 years
The 10-year DDFS rates for negative margins vs. positive margins for IC vs. positive margin for DCIS were 81.7% vs. 72.9% (HR 1.4; 95% CI 1.0–2.1; p = 0.038) vs. 79.8% (HR 1.5; 95% CI 0.9–2.5; p = 0.100).
In multivariate Cox regression analyses including the significant variables of the univariate analysis (BBC, tumour size, histology, positive lymph nodes, presence of in situ carcinoma, oestrogen receptor status, progesterone receptor status, lymph-angioinvasion, adjuvant systemic therapy and radiotherapy, and margin status) a positive margins for IC (HR 1.2; p = 0.223) or DCIS (HR 1.2; p = 0.602) did not show significance.
Disease-specific survival
≤ 40 years
The 10-year DSS rates for negative margins vs. positive margins for IC vs. positive margin for DCIS were 73.4% vs. 33.2% (HR 4.0; 95% CI 1.8–8.9; p = 0.001) vs. 77.8% (HR 0.9; 95% CI 0.2–4.0; p = 0.949) ().
Figure 3. The disease specific survival in 159 patients with 165 BCT according to margin status for women ≤40 years.
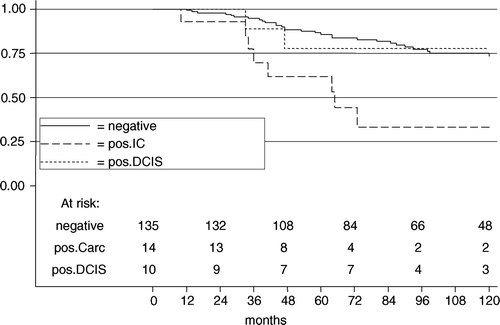
In multivariate Cox regression analysis including the significant variables of the univariate analysis, lymph-angioinvasion, BBC, and margin status, margin involvement for IC was significant (HR 2.8; 95% CI 1.1–6.7; p = 0.024). Margin involvement for DCIS showed no significance (HR 0.6; 95% CI 0.1–2.8; p = 0.555).
>40 years
The 10-year DSS rates for negative margins vs. positive margins for IC vs. positive margin for DCIS were 87.0% vs. 84.1% (HR 1.2; 95% CI 0.9–1.9; p = 0.374) vs. 76.7% (HR 1.9; 95% CI 1.1–3.2; p = 0.018) ().
Figure 4. The disease specific survival in 2 064 patients according to margin status for women >40 years.
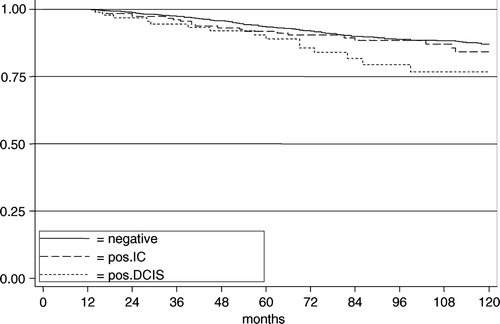
In multivariate Cox regression analyses including the significant variables of the univariate analysis (FH, tumour size, histology, positive lymph nodes, presence of in situ carcinoma, oestrogen receptor status, progesterone receptor status, lymph-angioinvasion, adjuvant systemic therapy and radiotherapy, and margins status) a positive margins for IC (HR 1.1; p = 0.653) nor DCIS (HR 1.2; p = 0.526) were significant.
Discussion
This study shows a diverse effect of a positive margin on outcome. The effect is not only dependent on whether the margin contains IC or DCIS, but also on the age of the patient. Young women, ≤40 years, a positive margin for IC results in a significantly higher local recurrence rate, distant metastasis rate, and lower survival. A positive margin for DCIS did not show these results, although this might be due to the small numbers. For women >40 years, a positive margin for DCIS is significantly related to a higher local recurrence rate and lower survival, although the latter is not significant in multivariate analyses. In contradistinction to young women a positive margin for IC in women >40 years does not result in a significant higher local recurrence rate or lower survival.
In this study we tried to establish the clinical relevance of a positive margin for DCIS in comparison to IC, as reported by the pathologist, on outcome. The value of this prospective study is that these are all patients from one region in The Netherlands. All patients were irradiated in a single institution, all pathology was performed in a single institution, and the treatment was relatively standardized during the years of this prospective study. Also, we present a rather large group of patients with positive margins compared to the published literature. On the other hand, due to the relative small number of women ≤40 years with positive margins the results have to be interpreted with caution.
In the eighties and early nineties grading of the pathology was not common in our region, resulting in a rather large percentage of patients without grading, which might be regarded as an omission of this study.
Randomized studies have established wide excision and radiation as an equal alternative to mastectomy in the treatment of breast cancer Citation[10–12]. The early studies mandated pathologically negative margins for those undergoing radiation. The absolute necessity of free margins is uncertain Citation[13]. The surgical margin status after breast-conserving surgery is considered mainly a strong predictor for local failure Citation[1–8], Citation[14–16]. In a recent review from Horst et al. they concluded that an adequate resection, as assessed by the status of the excisional margins on pathological examination, is a predictive factor with regard to local control Citation[17]. Although many papers have been published with regard to positive margins, most papers do not make a distinction between IC and DCIS.
Cowen et al. is one of the few who looked at positive margins for IC and DCIS in BCT, however the numbers are small Citation[14]. They analyzed 152 patients with positive margins, 39.7% IC, 41.1% DCIS, and 19.2% both. Similar to our results, they also found more local recurrences with margin involvement for DCIS (21.7%) as compared to invasive (15.5%), but the numbers are too small to reach statistical significance. They only concluded that margins with exclusively DCIS were not an indicator of a reduced relapse rate. Gage et al. showed that margin involvement for DCIS was a better indicator of local relapse than extensive intraductal carcinoma Citation[19]. Freedman et al. also looked separately at the invasive and in situ component, but could not establish a difference, although they did not make a distinction in age category Citation[20]. In 142 patients with a positive margin for invasive tumour and 49 for DCIS, they found 7% local recurrences in both categories.
Women ≤40 year are generally regarded as a separate entity in breast cancer with regard to prognosis and survival. Investigators from a number of centres have found that women younger than approximately 35–40 years at the time of diagnosis had a substantially higher risk of breast recurrence than older women Citation[17], Citation[18], Citation[21–26]. In earlier studies we showed age ≤40 years to be an important prognostic factor for local control Citation[24], Citation[27].
In the present analysis we also established a significant statistical interaction by age category, implicating the importance of analyzing the different age categories separately. Few studies also stress the importance of young age as a determinant factor for risk of local failure Citation[17], Citation[21], Citation[22], Citation[24]. Our study showed not only a significant relation of a positive margin for IC to local control, but also to distant metastasis and survival for young women in univariate and multivariate analyses. With regard to positive margin for DCIS in young women our analyses showed a possible trend to higher local recurrence rate. This study stresses the importance to have clear margins, in particularly for IC in women ≤40 years, not only in relation to local control but also in relation to distant metastasis. The reasons for different outcomes in relation to age are unclear; are we dealing with different tumours or is the endocrinological status of the young patient the reason?
In our analysis the incidence rate of local recurrence with positive margin for DCIS was 17.4% at 10-year in women >40 years, more than four times higher compared to negative margins. This is comparable to the local recurrence rate in the EORTC trial 10853 for a free margin status Citation[28]. This EORTC trial is one of the few studies in which the value of a positive margin for DCIS can be evaluated. One of the main conclusions of the trial was that the margin status was the most important factor in success for BCT. The NSABP trial B-17 also proved the radio sensitivity of DCIS Citation[29]. The aim of this trial was to test the hypothesis that local excision of DCIS followed by radiotherapy was more effective than lumpectomy alone. Free margins for DCIS are the predominant risk factor in women >40 years. Boyages et al. in a meta analyses on local recurrence in patients with DCIS confirmed margin status in BCT for DCIS to be a predictor of local recurrence Citation[30].
One of the questions which arises from this analysis is why we find a difference in local control with regard to positive margins for DCIS in comparison to IC? Is there a difference in histological growth patterns? Is DCIS in the breast a more extensive disease compared to IC? Holland et al. showed the extension of DCIS to be greater in comparison to IC Citation[31], Citation[32]. These findings are consistent with the hypothesis that positive margins for DCIS results in higher rate of local recurrence than do positive margins for IC using the same principles in designing the boost volume. Holland et al. also showed that DCIS does not have a multicentric distribution, meaning that a ‘one piece’ complete resection should be possible. However they found that in about 25% in their series tumours were very large. Incomplete resection might lead to a large residual tumour burden of DCIS. Re-excision with positive margin for DCIS should be more extensive and might even lead to an ablation.
Looking at the whole population we noticed also a negative outcome on distant metastasis of patients with a positive margin for IC. For the young patients the multivariate analysis showed significance with a HR 2.4, which is despite the small number important. Although for the older patients the multivariate analysis did not show significance the HR 1.4 at 10-years in univariate analysis is impressive looking at the large number of patients. Patients >40 years did not show a difference in local control, which might implicate that positive margins for IC is more a predictive factor for distant metastasis as for local recurrence.
We also noticed a relation between margin status and survival for the young patients. For patients >40 years the LRFS was significantly worse for positive margins for DCIS in women >40 years. This was translated in a significant worse survival with a HR of 1.9 for this group in univariate analysis, probably because of the increased local recurrence rate.
Conclusion
We showed a difference in outcome for positive margins for IC versus DCIS with regard to local control and also in relation to age. The impact of positive margin for IC seems to be limited to young women only, and is not only restricted to local control, but also to distant metastasis and survival. On the other hand a positive margin for DCIS is a risk factor for local control in women over 40 years. A positive margin for IC in this age category seems to have no impact on local control.
References
- Recht A. Selecting patients for breast-conserving therapy. Seminars in Breast Disease 2001; 4: 198–206
- Borger J, Kemperman H, Hart A, et al. Risk factors in breast-conserving therapy. J Clin Oncol 1994; 12: 653–60
- Schnitt SJ, Connolly JL. Pathological risk factors for local recurrence in patients with invasive breast cancer treated with conservative surgery and radiation therapy. Seminars in Breast Disease 1999; 2: 230–9
- Fourquet A, Campana F, Zafrani B, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: A 25-year follow-up. Int J Radiat Oncol Biol Phys 1989; 17: 719–25
- Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer 1995; 76: 259–67
- Kini VR, Vicini FA, Frazier R, et al. Mammographic, pathologic, and treatment-related factors associated with local recurrence in patients with early-stage breast cancer treated with breast conserving therapy. Int J Radiat Oncol Biol Phys 1999; 43: 341–6
- Renton SC, Gazet JC, Ford HT, et al. The importance of the resection margin in conservative surgery for breast cancer. Eur J Surg Oncol 1996; 22: 17–22
- Voogd AC, Peterse JL, Crommelin MA, et al. Histologic determinants for different types of local recurrence after breast-conserving therapy of invasive breast cancer. Eur J Cancer 1999; 35: 1828–37
- Stata/SE 8.2 for Windows. Stata Press; College Station; Stata Corporation: 2004.
- Liljegren G, Holmberg L, Bergh J, et al. 10-year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: A randomized trial. J Clin Oncol 1999; 17: 2326–33
- Early Breast Cancer Trialist’ Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomized trials. Lancet 2000;355:1757–70.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Eng J Med 2002; 347: 1233–41
- Taghian A, Mohiuddin M, Jagsi R, et al. Current perceptions regarding surgical margin status after breast-conserving therapy: Results of a survey. Ann Surg 2005; 24: 629–39
- Cowen D, Houvenaeghel G, Bardou V-J, et al. Local and distant failures after limited surgery with positive margins and radiotherapy for node-negative breast cancer. Int J Radiat Oncol Biol Phys 2000; 47: 305–12
- Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer. Cancer 2006; 106: 35–41
- Fredriksson I, Liljegren G, Palm-Sjovall M, et al. Risk factors for local recurrence after breast-conserving surgery. Br J Surg 2003; 90: 1093–102
- Horst KC, Smitt MC, Goffinet DR, et al. Predictors of local recurrence after breast-conservation therapy. Clin Breast Cancer 2005; 5: 425–38
- Wazer DE, Schmidt-Ullrich RK, Ruthazer R, et al. The influence of age and extensive intraductal component histology upon breast lumpectomy margin assessment as a predictor of residual tumor. Int J Radiat Oncol Biol Phys 1999; 45: 885–91
- Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer 1996; 78: 1921–8
- Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys 1999; 44: 1005–15
- Touboul E, Buffat L, Belkacémi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys 1998; 43: 25–38
- Veronesi U, Marubini E, Marubini L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann Oncol 2000; 12: 97–103
- Voogd AC, Neilsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomised trials. J Clin Oncol 2001; 19: 1688–97
- Jobsen JJ, van der Palen J, Meerwaldt JH. The impact of age on local control in women with pT1 breast cancer treated with conservative surgery and radiation therapy. Eur J Cancer 2001; 37: 1820–7
- Borg M F. Breast-conserving therapy in young women with invasive carcinoma of the breast. Aus Radial 2004; 48: 376–82
- Zhou P, Recht A. Young age and outcome for women with early-stage invasive breast carcinoma. Cancer 2004; 101: 1264–74
- Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH. The value of a positive margin for invasive carcinoma in breast conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys 2003; 57: 724–31
- Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma in situ; analysis of EORTC trial 10853. J Clin Oncol 2001; 19: 2263–71
- Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998; 16: 441–52
- Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ. A meta-analysis. Cancer 2000; 85: 616–28
- Holland R, Connely JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol 1990; 8: 113–8
- Holland R, Hendriks JHCL, Verbeek ALM, et al. Extent, distribution, and mammograhic/histological correlations of breast ductal carcinoma in situ. Lancet 1990; 335: 519–22
