To the Editor
The activity of bortezomib has been evaluated in large clinical trials on relapsed and/or refractory multiple myeloma (MM) Citation[1] but not on plasma cell leukemia (PCL), due to its relative rarity. So far, the effectiveness of bortezomib in PCL has only been supported by case reports Citation[2–4].
We report the hematological and clinical response to treatment with bortezomib in combination with dexamethasone in a 57-year-old woman with PCL. The disease had progressed both after front line combination chemotherapy based on vincristine, doxorubicine, dexamethasone (V.A.D.), which was followed by the onset of an intracranial localization that required radiotherapy, and after second line chemotherapy based on melphalan plus prednisone (M.P.).
The patient presented at our institution with a 13 month history of back-pain, weakness and progressive weight loss. In the period preceding our observation, she had undergone a left long stemmed total hip replacement arthroplasty, because of a fracture of the left femoral neck, without clinical improvement. Blood count analysis revealed anemia (hemoglobin 10.3 g/dl), a normal platelet count (platelets 169×109/l), leucocytosis (white blood cells 11.2×109/l), consisting mainly (56%) of plasma cells (6.2×109/l). Bone-marrow (BM) cytology showed 81% plasma cells infiltration and bone-marrow histology showed an interstitial infiltration pattern. Protein electrophoresis and immunofixation revealed 2.81 g/dl of monoclonal immunoglobulin (MI) Gλ in the serum and 1180 mg/l free lambda (λ) chains in the urine.
In the serum, the levels of calcium (8.8 mg/dl) (NR: 8–10.5 mg/dl) and alkaline phosphatase (ALP) (86 U/l) (NR: 50–136 U/l) were normal; β-2 microglobulin (B2M) was 3.24 mg/l (NR: 0.70–1.80 mg/l) and C-reactive protein (CRP) was 3 mg/dl (NR: up to 0.30 mg/dl). A radiological skeletal survey showed multiple bone lesions (>3) in the skull, spine, hip, and ribs. The diagnosis of PCL was made (). No HLA compatible siblings were found and the patient was treated with the V.A.D. chemotherapy.
Table I. Patient's clinical evolution according to therapy.
After four courses, in spite of a decrease of the MI Gλ in the serum, free λ chains in the urine and plasma cells in peripheral blood, the patient showed an oedema of the upper right eyelid spreading up to the right temporal region. A cervicofacial computed tomography (CT) scan showed an intracranial mass highly suspected to be of a myelomatous nature (). This mass spread from the anterior part of the right temporal bone and the greater wing of the right sphenoid bone, up to the right temporal fossa and the middle cranial fossa. Moreover, it caused a partial destruction of the lateral wall of the right orbit without intra-orbital extension.
Figure 1. Craniofacial computed tomography (CT) scan demonstrating an intracranial mass in our PCL patient.
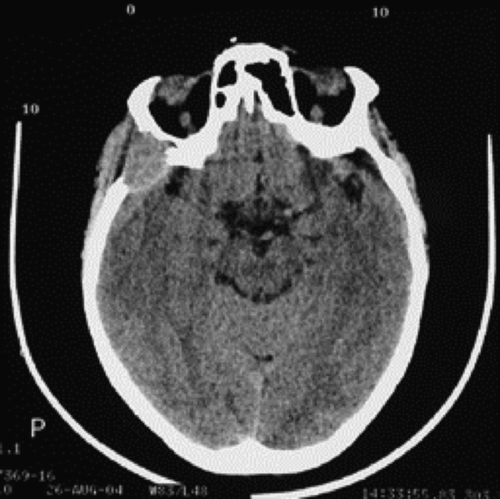
At this stage, the administration of V.A.D. was suspended. The patient underwent external radiotherapy (total dose 24 Gy) on the temporal region and obtained a clinical improvement despite a persistent intracranial mass. Due to the progressive increase of the levels of the MI Gλ in the serum and the free λ chains in the urine, pulse doses of oral M.P. were administered for four courses.
Due to the poor response and the significant hematological toxicity [grade 3 according to World Health Organization (WHO) toxicity scale], the administration of M.P. was stopped and the patient was treated with bortezomib (B) 1.3 mg/m2/ intravenously (i.v.) on days 1, 4, 8 and 11, plus dexamethasone (D) 20 mg i.v. on days 1, 2, 4, 5, 8, 9, 11, 12 every 3 weeks for four courses and every 5 weeks for further four courses.
No tumor lysis syndrome was observed. The patient experienced a transient hematological toxicity (thrombocytopenia grade 2 WHO), which returned to baseline in the period between treatment cycles, as well as reversible peripheral neuropathy (grade 1 WHO).
By the 2nd cycle of B.D., her performance status (PS) had improved. At the end of the 8th cycle of B.D., the disease was considered to be in partial remission in accordance with published disease response evaluation criteria Citation[5]. A craniofacial CT-scan demonstrated a decrease in the intracranial mass. No new osteolytic lesion was demonstrated by a new skeletal survey. In addition, ALP serum level, even if within the normal laboratory range, had increased by 22% from the baseline value to 110 U/l.
The patient was considered eligible for mobilization of peripheral blood stem cells followed by an autologous transplant that she refused. The remission still persists at 35 months after diagnosis.
The case presented in this report seems to be a secondary PCL after an unrecognised MM that could have acquired such aggressive characteristics as to progress, apart from the known leukemic spread, with osteolytic lesions and extramedullary involvement, both refractory to conventional treatment.
In our case, treatment with B.D. had a crucial impact on the clinical outcome, blocking further progression of the disease, without severe hematological and non-hematological toxicity. Despite the patient's refusal of an autotransplant, her survival is comparable with the median survival reported in a series of primary PCL patients treated with stem cell transplantation Citation[6] and is unusually long if we consider a series of PCL patients treated with conventional chemotherapy. In fact, in these cases the median survival is of about 6 months for primary PCL and only 2 months for secondary PCL Citation[7].
Due to the onset of an intracranial extramedullary mass, local radiotherapy was preferred as up-front treatment for this complication. The treatment with B.D., later performed, could have had a synergic role in the achievement of the regression of the same mass.
After the combination therapy of bortezomib and dexamethasone, no new osteolytic lesions were demonstrated in our case. In addition, the increase of ALP, a marker of osteoblastic activity, was associated with achievement of the response. This result is in line with those of other studies Citation[8] in which the response to bortezomib has been suggested to be related with osteoblastic activation.
Our report shows that combination therapy of bortezomib and dexamethasone was effective in the treatment of our PCL patient, with progressive disease after conventional therapy. The inclusion of bortezomib in the initial treatment of large series of PCL patients may be of interest to evaluate the impact of this drug on disease-free and overall survival.
References
- San Miguel J, Bladé J, Boccadoro M, Cavenagh J, Glasmacher A, Jagannath S, et al. A pratical update on the use of bortezomib in the management of multiple myeloma. The Oncologist 2006; 11: 51–61
- Esparis-Ogando A, Alegre A, Aguado B, Mateo G, Gutierrez N, Blade J, et al. Bortezomib is an efficient agent in plasma cell leukemias. Int J Cancer 2005; 114: 665–7
- Jaskiewicz AD, Herrington JD, Wong L. Tumor lysis syndrome after bortezomib therapy for plasma cell leukemia. Pharmacotherapy 2005; 25: 1820–5
- Grassinger J, Sudhoff T, Andreesen R, Hennemann B. Complete remission and successful stem cell mobilization after treatment of refractory plasma cell leukemia with bortezomib. Ann Hematol 2006; 85: 132–3
- Bladé J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response in patients with multiple myeloma treated with high-dose therapy and hemopoietic stem cell transplantation. Br J Haematol 1998; 102: 1115–23
- Saccaro S, Fonseca R, Veillon DM, Cotelingam J, Nordberg ML, Bredeson C, et al. Primary plasma cell leukemia: Report of 17 new cases treated with autologous or allogeneic stem-cell transplantation and review of the literature. Am J Hematol 2005; 78: 288–94
- Jimenez-Zepeda VH, Dominguez VJ. Plasma cell leukemia: A rare condition. Ann Hematol 2006; 85: 263–7
- Zangari M, Esseltine D, Lee CK, Barlogie B, Elice F, Burns MJ, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol 2005; 131: 71–3
