Abstract
A phase I trial of short-time oxaliplatin (E), capecitabine (X) and epirubicin (E) for patients with metastatic gastric cancer was initiated to establish the recommended dose for further therapy with short-time EXE. Patients received out-patient therapy with a fixed dose of epirubicin 50 mg/m2 day 1; escalating doses of capecitabine (1 000 to 1 250 mg/m2/day continuously) and escalating doses of oxaliplatin (85 to 130 mg/m2 day 1 as a 30 minutes infusion). Cycles were repeated every 21 days for a maximum of 8 cycles. From June 2003 to June 2004, 31 patients were treated. Median age was 61 years (39–75 years), and median performance status was 0 (0–2). At level 3, one of six patients developed DLT and at dose level 4, two of six patients developed DLT (both patients had grade 4 hematological toxicities) and thus further dose escalation was not attempted. Median number of cycles was 6 (1–8), median survival was 9.2 months and median TTP was 7.5 months. A combination of epirubicin 50 mg/m2 day 1, capecitabine 1 000 mg/m2 continuously and oxaliplatin 130 mg/m2 day 1 each 3 weeks is an easily administered and well tolerated out-patient regimen for patients with non-resectable gastric cancer.
Patients with gastric adenocarcinoma have a poor prognosis, with an overall survival of approximately 10% at 5 years in Denmark Citation[1]. Although the incidence is declining, it is the fifth most common cancer in Europe Citation[2]. An estimated 171 000 new cases of gastric cancer will be diagnosed in Europe yearly Citation[2]. There is no chemotherapy regimen considered to be standard treatment for advanced gastric cancer worldwide but in Europe ECF (Epirubicin 50 mg/m2 day 1, cisplatin 60 mg/m2 day 1 and continuous infusion of 5-fluorouracil (5-FU) 200 mg/m2 daily) is widely used. Several randomized studies have established that combination chemotherapy prolongs survival and improves quality of life in patients with metastatic gastric cancer Citation[3–5]. A decade ago FAMtx was often considered to be standard therapy Citation[6], but a large randomized study Citation[7], Citation[8] proved that ECF significantly improved response rate and survival and therefore ECF is presently considered by many as reference therapy Citation[8]. However, it requires continuous intravenous infusion of 5-FU through a central venous catheter, which is associated with significant morbidity, particularly venous thromboses and infections Citation[9], Citation[10]. We sought to develop a regimen that is comparable in efficacy with ECF, but which can easily be administered in the out-patient setting. Capecitabine is an orally available prodrug which is converted to 5-FU selectively in tumours Citation[11]. Consequently, replacing the continuous infusion of 5-FU by capecitabine simplifies the administration and overcomes the need for a central venous catheter. Cisplatin is nefrotoxic, ototoxic, severely emetogenic and requires i.v. hydration and therefore we substituted cisplatin with oxaliplatin. Oxaliplatin has in combination with 5-FU in several phase II studies shown efficacy with high response rates, promising median overall survival and a favourable toxicity profile Citation[12–15]. The dose limiting toxicity of oxaliplatin is cumulative peripheral neurotoxicity, and to reduce neurotoxicity it is recommended to give oxaliplatin as a 120 min infusion Citation[15]. However, in a former pilot study and in a former phase II trial in patients with colorectal cancer, we administered oxaliplatin as a 30 min infusion without extra neurotoxicity Citation[16], Citation[17]. In this phase I study we therefore wanted to administer short-time oxaliplatin in combination with capecitabine and epirubicin (EXE) to establish a new well-tolerated out-patient regimen.
Patients and methods
Patient selection
Patients were eligible if they had histologically confirmed non-resectable adenocarcinoma of the stomach or gastrooesophageal junction. Eligibility criteria included estimated life expectancy > 12 weeks; measurable or non-measurable disease; adequate renal function (calculated creatinine clearance > 30 ml/min by the Cockroft and Gault formula); adequate hepatic function (serum bilirubin < 1.5×upper normal limit (UNL); transaminases < 3×UNL, however in cases of liver metastases, there were no upper limit for transaminases); adequate hematological function (neutrophil count > 1.5×109/l; platelets > 100×109/l); no prior chemotherapy; WHO performance status 0 to 2, and age between 18 and 75 years. Other inclusion criterias were ability to tolerate and comply with oral medication, no signs of intestinal obstruction or malabsorption, no peripheral neuropathy, no co-existent severe medical illness, no sign of brain metastases and no concomitant treatment with other anticancer therapy.
The study was approved by the local ethics committee and Danish health authority and signed informed consent was obtained before study entry.
Study design and treatment
Patients were planned to receive escalating doses of oxaliplatin (85 to 130 mg/m2) as a 30 min. i.v. infusion on day 1; escalating doses of oral capecitabine (1 000 to 1 250 mg/m2 divided in two daily doses continuously); and a fixed dose of epirubicin (50 mg/m2) as a 20 min. i.v. infusion on day 1 (). Treatment was repeated every 3 weeks for up to a maximum of eight cycles of treatment unless stopped before because of disease progression or unacceptable toxicity.
Table I. Dose escalation scheme
Sequential cohorts of patients were treated at progressively higher dose levels. A minimum of three patients were entered at each dose level, with an additional three patients added if one of the first three patients experienced a dose limiting toxicity (DLT) during the first course of treatment. Dose escalation was continued if 0/3 or 1/6 experienced DLT. While waiting for patients to complete their first cycle additional patients were offered EXE at the actual dose level.
Evaluation of toxicity
Toxicity was graded using the National Cancer Institute Common Toxicity Criteria (NCI CTC v2.0). Dose escalation, determination of DLT and maximum tolerated dose (MTD) was on the basis of toxicity from the first cycle of EXE only.
DLT was defined as the occurrence of any of the following toxicities during the first cycle of EXE: any grade 3/4 non-hematological toxicity other than alopecia, febrile neutropenia, grade 4 thrombocytopenia (platelet count < 25×109/l) or grade 4 neutropenia (absolute neutrophil count < 0.5×109/l).
The MTD was defined as the dose level that produced DLT in at least two of the first three patients or in at least two of six patients. The recommended dose (RD) was defined as the dose level that is one level below MTD.
Patient evaluation
Physical examination, assessment of medical history, evaluation of performance status, toxicity, complete blood counts, and biochemical tests were performed at baseline and prior to each cycle of therapy. Presence of measurable lesions was not required in this study, but all patients had an abdominal and/or thoracic CT-scan performed at baseline and after every three cycles of therapy to assess tumour response or exclude progression. Responses were recorded according to RECIST criteria. After the end of treatment, patients were followed every 3 months until progression or death.
Statistical methods
Non-parametric statistics were applied. All median values are followed by range in brackets. After cessation of treatment patients without documented progression were followed every 3 months with clinical and radiological evaluation. Time to progression (TTP) and overall survival (OS) was updated as at December 1, 2005. OS and TTP were generated according to the Kaplan-Meier method. Data were recorded and analysed in a Medlog®database. All analyses were done on an intention to treat population.
Results
Patient characteristics
Between June 2003 to June 2004, 31 patients were treated at two Danish university hospitals. Patient characteristics at baseline are summarized in . The median age was 61 years (range 39–75). Performance status was zero in 16 patients, one in 13 patients, and two in two patients. Twenty patients had adenocarcinoma located in cardia, and 11 patients had adenocarcinoma located in corpus or antrum of the stomach. No patients had previously received chemotherapy or radiotherapy.
Table II. Patient characteristics at baseline
Four dose levels were studied (). Median number of cycles per patient was 6 (range of 1–8); 12 (39%) patients completed seven or eight courses of therapy. Median dose of oxaliplatin was 620 (100–920) mg/m2. Ten patients were treated at dose level IV, receiving a median of five courses.
Toxicity
A total of 31 patients were treated at four dose levels in a 21-day cycle (). No DLT was seen during the first cycle of dose levels I and II. At dose level III, one of three patients developed DLT (infection without neutropenia) () and three more patients were included at this dose level without any DLT. At dose level IV, one of three patients developed DLT (febrile neutropenia) and additionally one of the next three patients (grade 4 neutropenia without fever). Therefore, in accordance with the study design, the MTD had been reached at level IV, and further dose escalation was not performed. However, four additional patients received therapy at dose level IV without DLT. Therefore the protocol committee recommended using dose level IV for the ongoing phase II study.
Table III. Grade 2–4 toxicity after 1st cycle of EXE, (n = 31)
Worst toxicity for all patients and all cycles are listed in . Multiple toxicities in the same patients are scored as separate events. Neutropenia was the principal hematologic toxicity of the combination, 31% of patients experienced grade 2 neutropenia, and 16% grade 3–4 neutropenia, but only one patient (3%) had grade 4 febrile neutropenia. Grade 4 toxicities were rare. Two patients had neutropenia grade 4 and one patient had febrile neutropenia grade 4. No patient experienced non-hematological toxicity grade 4. Of the 31 patients, grade 3–4 toxicities included neutropenia (16%), infection without neutropenia (6%), PPE (6%), thrombocytopenia (3%), febrile neutropenia (3%), diarrhea (3%), nausea (3%), vomiting (3%) and neuropathy (3%). Only one patient (3%) experienced peripheral neuropathy grade 3, and seven patients (23%) experienced peripheral neuropathy grade 2. There was no case of laryngopharyngeal dysaesthesia (LPD) and there was no treatment-related death in this study.
Table IV. Worst toxicity after median 6 cycles of EXE
Efficacy
The reasons for discontinuation of therapy were completion of therapy (n = 12), progressive disease or deterioration of health (n = 9), toxicity (n = 6), patients refusal (n = 2) and other (n = 2).
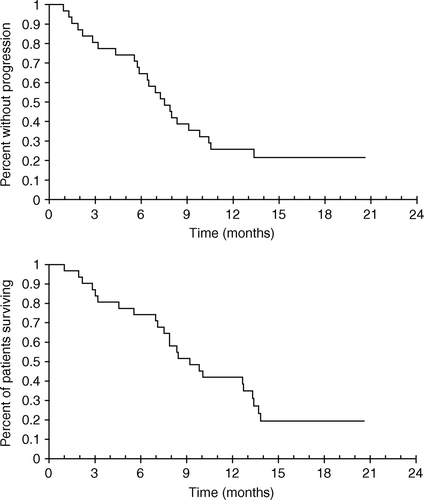
Twenty-one patients had measurable disease. No patient had complete response but nine (42%) patients obtained partial response. Only four patients had progressive disease at the first radiological evaluation. Median survival was 9.2 months and median time to progression was 7.5 months ().
Discussion
The primary aim of this study was to determine dose limiting toxicity, and to establish the recommended dose for a phase II study. Dose limiting toxicities were primarily neutropenia. At level III one patient had infection without neutropenia, and at level IV two patients had febrile neutropenia and garde 4 neutropenia without fever, respectively. According to the protocol the MTD had been reached at dose level IV because two of six patients had DLT. In other phase I studies the MTD was defined as DLT in three of six patients Citation[20], Citation[21]. In our study, we additionally treated four patients at dose level IV without DLT, and we therefore decided to recommend dose level IV for the phase II study.
Grade 3–4 toxicities included neutropenia (16%), infection without neutropenia (6%), PPE (6%), febrile neutropenia (3%), thrombocytopenia (3%), diarrhea (3%), nausea (3%), vomiting (3%) and neuropathy (3%). Compared to the toxicities reported in the phase III study by Webb et al. Citation[7], Citation[8], the ECF regimen induces more neutropenia (36%), nausea/vomiting (17%) and infections (8%). In contrast, peripheral neuropathy is a major problem in patients receiving EXE.
Despite the short infusion time, no patients experienced laryngeal dysaesthesia and short-time infusion of oxaliplatin in this combination seems safe.
In the 21 patients with measurable disease, the response rate was 42%, with a median survival of 9.2 months. The response rate of EXE in this study seems comparable to the response rate of 45% reported with the ECF regimen Citation[7], Citation[8].
The REAL 2 trial Citation[18] is a phase III 2×2 design study comparing ECF with epirubicin, cisplatin, capecitabine (ECX), epirubicin, oxaliplatin, 5-FU (EOF); and epirubicin, oxaliplatin, capecitabine (EOX) in patients with advanced oesophagogastric cancer. The first interim analysis was carried out after inclusion of 80 patients to compare fluoropyrimidine related toxicity. Grade 3–4 fluoropyrimidine related toxicity in patients receiving capecitabine 500 mg/m2 b.i.d. was only 5%, compared to 17% of the patients receiving infused 5-FU and therefore the capecitabine dose was increased to 625 mg/m2 b.i.d. according to the protocol. A second interim analysis was carried out after inclusion of 204 patients to evaluate toxicity and response rates. The response rate of the ECF regimen was 31% but 48% in patients receiving EOX (comparable to EXE). In patients receiving capecitabine 625 mg/m2 grade 3/4 fluoropyrimidine toxicity was 14.7% which was equivalent to the grade 3–4 fluoropyrimidine toxicity of 13.7% in the patients receiving infused 5-FU 200 mg/m2. Comparing non-fluoropyrimidine toxicity in patients treated with capecitabine 500 mg/m2 b.i.d. (EOX 500) versus capecitabine 625 mg/m2 b.i.d. (EOX 625), there was more nausea, lethargy, infection, febrile neutropenia and especially peripheral neuropathy in the patients treated with capecitabine in the high dose, but again the patients number is small, and we must await the final results from the ongoing phase III study before any final conclusions can be made.
Also in this issue Idelevich et al. Citation[22] reported a II study into the efficacy and toxicity of PELUF (in contrast to the EXE regimen capecitabine were substituted with UFT/leucovorin) and the authors reported almost identical efficacy (response rate 38%, TTP 6.5 months and OS 9.5 months) and good tolerability.
From a practical perspective EXE is preferable to ECF because it can be administered in the out patient setting resulting in a greater patient and hospital convenience.
Conclusion
In conclusion a combination of epirubicin 50 mg/m2 day 1, oxaliplatin 130 mg/m2 day 1 and capecitabine 500 mg/m2×2 daily continuously each 3 weeks, is an easily administered and well tolerated regimen, and is recommended for our phase II study. In this combination oxaliplatin can safely be given as a 30 min infusion. The ease and convenience of EXE makes it a possible candidate for a new reference therapy.
References
- Coleman MP, Gatta G, Verdecchia A, et al. Eurocare-3 summary: Cancer survival in Europe at the end of the 20th century. Ann Oncol 2003; 14(Suppl 5)128–49
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005; 16: 481–8
- Janunger KG, Hafstrom L, Nygren P, et al. A systematic overview of chemotherapy effects in gastric cancer. Acta Oncol 2001; 40: 309–26
- Hill ME, Cunningham D. Medical management of advanced gastric cancer. Cancer Treat Rev 1998; 24: 113–8
- Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8: 163–8
- Wils JA, Klein HO, Wagener DJ, et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin–a step ahead in the treatment of advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J Clin Oncol 1991; 9: 827–31
- Waters JS, Norman A, Cunningham D, et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: Results of a randomized trial. Br J Cancer 1999; 80: 269–72
- Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15: 261–7
- Findlay, M, Cunningham, Norman, A, et al. A phase II study in advanced gastro-oesophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF). Ann Oncol 1994;5:609–16.
- Evans TR, Lofts FJ, Mansi JL, Glees JP, Dalgleish AG, Knight MJ. A phase II study of continuous infusion 5-fluorouracil with cisplatin and epirubicin in inoperable pancreatic cancer. Br J Cancer 1996; 73: 1260–4
- Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998; 34: 1274–81
- Louvet C, Andre T, Tigaud JM, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 2002; 20: 4543–8
- Al-Batran SE, Atmaca A, Hegewisch-Becker S, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 2004; 22: 658–63
- Chao Y, Yeh KH, Chang CJ, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 2004; 91: 453–8
- Cvitkovic E, Bekradda M. Oxaliplatin: A new therapeutic option in colorectal cancer. Semin Oncol 1999; 26: 647–62
- Pfeiffer P, Hahn P, Jensen HA. Short-time infusion of oxaliplatin (Eloxatin) in combination with capecitabine (Xeloda) in patients with advanced colorectal cancer. Acta Oncol 2003; 42: 832–6
- Pfeiffer P, Sorbye H, Ehrsson H, et al. Short-time infusion of oxaliplatin in combination with capecitabine (Xelox30) as second line therapy in patients with advanced colorectal cancer after failure to irinotecan and 5-fluorouracil. Ann Oncol 2006; 33: 70–4
- Sumpter K, Harper-Wynne C, Cunningham D, et al. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer 2005; 92: 1976–83
- Hong YS, Song SY, Lee SI, et al. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol 2004; 15: 1344–7
- Tsai JY, Iannitti D, Berkenblit A, et al. Phase 1 study of docetaxel, capecitabine, and carboplatin in metastatic esophagogastric cancer. Am J Clin Oncol 2005; 28: 329–33
- Ychou M, Conroy T, Seitz JF, et al. An open phase 1 study assessing the feasibility of the triple combination: Oxaliplatin plus irinotecan plus leucovorin/5-fluorouracil every 2 weeks in patients with advanced solid tumors. Ann Oncol 2003; 14: 481–9
- Idelevich, E, Karminsky, N, Dinerman, M, et al. Phase II study of cisplatin, epirubicin, UFT, and leucovorin (PELUF) as first-line therapy in metastatic gastric cancer. Acta Oncol, (in press).