Abstract
Based on randomized studies bone-marrow supported (BMS) high-dose chemotherapy (HDCT) is not superior to conventional CT as adjuvant treatment for high-risk breast cancer. To compare the cost-effectiveness of these treatments we examined the data of Finnish patients in the SBG9401 trial Citation. Patients were randomized to receive either dose-escalated (de FEC) (group A, n =59) or FEC and HDCT+BMS (group B, n =70). They received adjuvant radiotherapy (RT) + tamoxifen. All direct health care costs of first line treatment at the oncology units were considered as well as productivity costs within the first 3 years of follow-up. Effectiveness was measured by the number of survival days during 5 years of follow-up. The mean direct health care costs were significantly higher in group B (25 829 € in group A vs. 36 605 € in group B, p <0.001), mainly due to a higher number of hospital days. Half of the costs in group A was due to the use of filgrastim (15 335 € in A and 2969 € in B, p <0.001). The costs of RT were only 5% of total costs. There was no statistically significant difference between the groups in the number of survival days, but sensitivity analysis based on bootstrapping suggested that treatment A would be a less costly and more effective alternative in a great majority of cases.
Breast cancer is the most common cancer in the Western world and cancer has been estimated to account for about 11% of the costs of all illnesses in the United States in 1985. The National Institutes of Health estimated that the costs of cancer in 2002 were US $171.6 billion of which US $60.9 billion were attributed to direct medical costs Citation[2]. According to data from 2001, breast cancer accounts for 15–20% of all cancer costs and 1% of the total health budget in the USA Citation[3]. Primary therapy includes surgery, chemotherapy, endocrine therapy and radiotherapy. The last-mentioned approach is effective and relatively cheap when compared to chemotherapy Citation[4]. The same modalities are used for breast cancer relapses and more recently the monoclonal antibody trastuzumab, chemotherapy and/or radiotherapy in advanced stages. In an analysis of over 2000 patients the costs of initial treatment were US $10 813 and of terminal care US $17 686 per patient in 1992 Citation[3]. The total costs of initial care increased with disease stage. The direct costs of diagnosis and initial treatment of a cohort of 17 700 patients diagnosed in 1995 are estimated to be 8014–8225 CAD per patient Citation[5]. Life-time cost modeling of breast cancer has shown that especially the treatment of metastatic breast cancer constitutes a substantial economic burden to the health care system Citation[6–8]. In addition metastatic breast cancer is in most cases incurable and very costly due to the increasing use of chemotherapy Citation[9]. The main means to increase survival and reduce costs is an early diagnosis and effective initial treatment, including adjuvant treatment.
A recent health economic analysis comparing conventional-dose chemotherapy with high-dose chemotherapy supported by autologous hematopoetic stem-cell transplantation in patients with metastatic breast cancer showed high-dose therapy to increase both morbidity and costs with no improvement in survival Citation[10]. In their analysis of 180 women treated within the context of a randomized study, patients in the transplantation group were hospitalized more often, and the costs in this group were US $85 055 per patient vs. US $28 169 in the conventional chemotherapy group.
With a view to increasing disease-free survival, high-dose chemotherapy with stem cell support was evaluated in the 1990s in many large prospective randomized clinical trials in an adjuvant setting in patients with high-risk breast cancer Citation[1], Citation[11–14]. None of the marrow-supported high-dose studies demonstrated overall survival improvement. It has however been debated whether patients with ten or more positive lymph nodes would benefit from high-dose chemotherapy with stem cell rescue Citation[12], Citation[15], Citation[16].
In the Scandinavian countries a prospective randomized adjuvant trial was initiated in 1994, and 524 high-risk patients were randomized to receive either high-dose chemotherapy with peripheral stem cell support or a tailored FEC regimen Citation[1], Citation[17]. In this study we compare the cost-effectiveness of these two treatment modalities in order to improve the cost-effectiveness of future adjuvant therapies.
Patients and methods
Altogether 524 high-risk patients were randomized in a Scandinavian Breast Cancer Study Group study, SBG9401, from July 1994 to 1997 in four Nordic countries. The results of early analysis have been published in the Lancet in 2000 Citation[1].
This analysis is based on prospectively collected data of patients treated at three main Finnish centers taking part in that trial (n = 129, 24.6% of the total 524 patients, 1). Fifty-nine patients (group A) were randomized to receive nine cycles of tailored dose escalated FEC every third week (5-fluorouracil starting dose 600 mg/m2, epirubicin 75 mg/m2 and cyclophosphamide 900 mg/m2 q 3 weeks, the highest dose level was 5-fluorouracil 600 mg/m2, epirubicin 120 mg/m2 and cyclophosphamide 1800 mg/m2) supported by granulocyte colony-stimulating factor, filgastim 5 µg/kg, Neupogen® (Amgen, Basel, Switzerland) and prophylactic antibiotics. In group B, 70 randomized patients received three cycles of standard FEC followed by HDCT with CTCb (6000 mg/m2 cyclophosphamide, 500 mg/m2 thiothepa, 800 mg/m2 carboplatin, all drugs being given as continuous intravenous infusion over 96 h on days −7 to −4) with PSCT (at least 2×106 CD34+ cells/kg, transfused on day 0) Citation[1]. All patients received adjuvant radiotherapy and tamoxifen for 5 years. Two patients, one in each group, did not receive the trial medication and were excluded from the health economic analyses. In the original study (n = 524) and in the present Finnish subgroup patients (n = 129) there were no statistically significant differences between the trial groups in the other prognostic features and the mean age of the patients in these Finnish patient groups at the time of diagnosis was 49 years (range 41–59 years, no significant difference between the groups).
All health care resource use in the respective oncological units during adjuvant therapy (hospitalization, drugs, transfusions, growth factors, antibiotics, laboratory tests, radiological examinations etc.) was counted for each patient from the hospital records and valued at the unit costs in 1996 in these hospitals. The costs of surgery and adjuvant tamoxifen for 5 years were not included in the analysis because they were same between the two groups and only the expenses at the oncology unit (radiotherapy and chemotherapy) were considered. The direct costs cover the first line treatment during the first 3 years of follow-up. The sick leave days during the same period were valued at the mean gross salary (+35% social security expenses) of women in Finland in 1996 to estimate the productivity costs. The sum of direct health care costs and productivity costs is referred to as total costs.
Effectiveness was measured by the number survival days within the follow-up period of 5 years. Costs and health benefits were not discounted due to a short follow-up period and similar time pattern of the occurrence of costs and health benefits between the treatments.
The prospective clinical trial SBG 9401 was approved by the ethics committees of the University Hospitals of Helsinki, Tampere and Turku, and written informed consent was obtained from every patient.
Statistical analysis
Data analysis was carried out using SPSS 13.0 Citation[18] statistical programs. Fisher's exact test and χ2 test were used to test the significance in the cross-tabulated data. Independent samples t-test was used to test differences between the groups in continuous variables. All reported p-values are two-sided. Probability values of <0.05 were considered statistically significant. To assess uncertainty, 10 000 resamples from the original cost-effectiveness data set were simulated using a bootstrapping technique. Results are given as mean incremental costs and effects with their 95% confidence intervals (CI), incremental cost-effectiveness ratio, cost-effectiveness plane and cost-effectiveness acceptability curve.
Results
The breakdown of costs is shown in . The mean direct health care costs of the high-dose adjuvant treatment were significantly higher in group B (25 829 € in group A vs. 36 605 € in group B, p < 0.001, ). The main reason for the higher costs in group B was the higher number of hospital days. The lowest total cost (22 047 €) for an individual patient was noted in group A, the highest (52 451 €) in group B.
Figure 1. Box plot figures for direct health care costs between the two treatment groups. The boxes indicate the lower and upper quartiles and the central spot is the median. The upper end of the bar is the maximum and the lower end the minimum value.
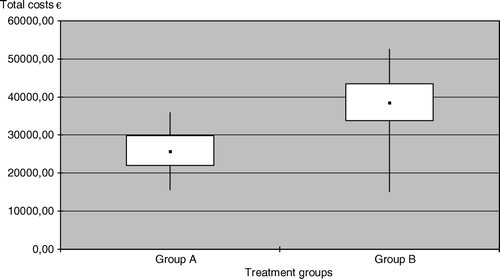
Table I. Mean costs by items in different treatment groups, A = dose-escalated FEC and B = high-dose chemotherapy with peripheral stem cell support (standard deviation in parentheses).
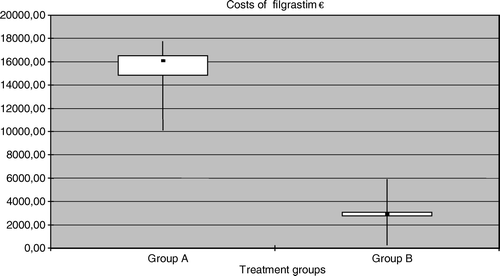
In group A all 58 patients received prophylactic antibiotics during each chemotherapy cycle at a mean cost of 511 € (range 119–597 €), whereas in group B the corresponding mean cost was only 53 € (range 0–1791 €), as prophylactic antibiotics were used orally during the mobilization chemotherapy, p < 0.001. The costs of filgrastim accounted for half of the total drug costs in group A. The mean cost of filgrastim was significantly higher in group A (15 334 €) than in group B (2969 €, p < 0.001, ).
Figure 2. Box-plot figures for the costs of the use of leukocyte growth factor between the groups. The boxes indicate the lower and upper quartiles and the central spot is the median. The upper end of the bar is the maximum and the lower end is the minimum value.
The costs of radiotherapy were quite similar in both groups (1687 € in group A and 1572 € in group B, p = 0.047), accounting for only 5% of the direct health care costs.
There was no statistically significant difference in the productivity costs, since there was no statistically significant difference in the number of sick leave days, the mean being 456 days in group A (range 170–1261 days, SD 283) and 419 days in group B (range 141–528 days, SD 343), p = 0.516, despite the fact that duration of therapy was significantly longer in group A.
At 5-year follow-up there was no statistically significant difference between the groups in the mean number of survival days, p = 0.330. A cost-effectiveness comparison based on direct health care costs and survival days suggests that treatment A is weakly dominant, that is, it produces the same effectiveness at a lower cost. A comparison based on total costs suggests that there is no difference between the treatments in cost-effectiveness.
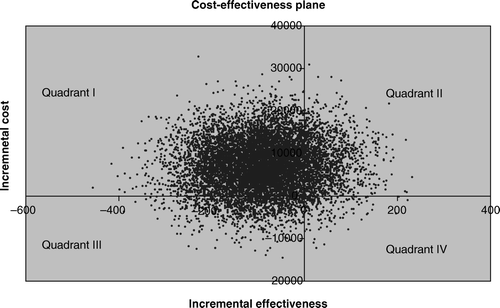
Bootstrapping showed that when costs were measured by direct health care costs the mean incremental cost of treatment B was 10 763 € (8083–13 418), and that in 84% of the cases treatment A was both less costly and more effective and in 16% less costly and less effective (). When costs were measured by total costs the mean incremental cost of treatment B was 7095 € (−4332–18 679 €), and the percentage of cases where treatment A was less costly and more effective fell to 74%. In only 2% of cases would treatment A be more costly and less effective than treatment B (). Mean incremental effect of treatment B was −88 days (−263–86).
Figure 3. The cost-effectiveness plane with direct health rate costs (vertical axis = incremental direct health care cost, €; horizontal axis = incremental effectiveness, survival days gained). In 84% of simulated cases treatment B was both more costly and less effective (Quadrant I), and in 16% more costly, but more effective (Quadrant II).
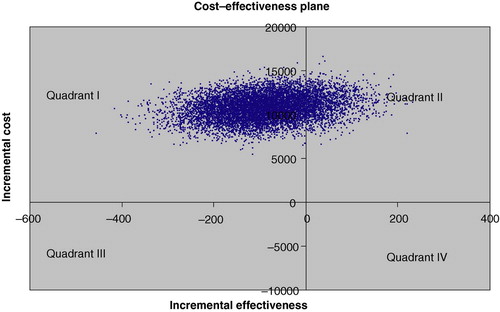
Figure 4. The cost-effectiveness plane with total costs (vertical axis = incremental total cost, €; horizontal axis = incremental effectiveness, survival days gained). In 74% of simulated cases treatment B was both more costly and less effective (Quadrant I), in 14% more costly and more effective (Quadrant II), in 10% less costly and less effective (Quadrant III), and in 2% of cases less costly and more effective (Quadrant IV).
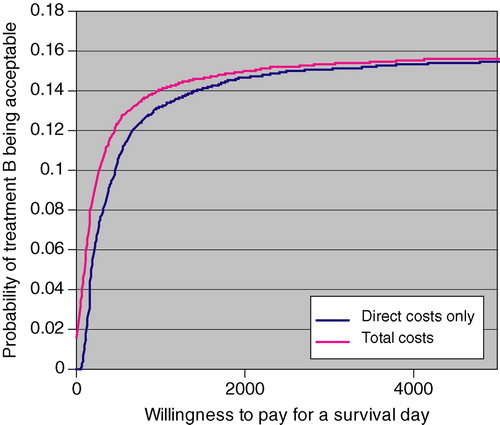
Cost-effectiveness acceptability curves of treatment B are shown in . Even if the willingness to pay for an extra survival day would be as high as 5000 € (1.8 million € per life year gained), only in less than 16% of cases would treatment B be acceptable, that is, the cost per survival day, be it measured by direct health care costs or total costs, would be less than 5000 €.
Discussion
Breast cancer is the most common cancer among women in Western countries, where adjuvant chemotherapy in intermediate and high-risk patients is well established Citation[1], Citation[19] The use of aggressive cytotoxic chemotherapies with stem cell support as adjuvant treatment of high-risk breast cancer patients was actively studied during the 1990s Citation[1], Citation[12–14], Citation[20]. However, none of the high-standard large randomized studies revealed overall survival improvement with marrow-supported HDCT as against more conventional treatment modalities.
The goal of HDCT with PSCT is to achieve good treatment response and thus to improve the long-term prognosis of several malignancies Citation[21]. In the past high-dose chemotherapy supported by peripheral stem cell transplantation has proved to be at least as effective as treatment supported by bone-marrow transplantation, while costs are lower and side-effects fewer Citation[22–25]. Due to the high costs of bone marrow-supported intensive chemotherapy many studies have analyzed the mobilization of peripheral stem cells and compared the cost-effectiveness of autologous bone-marrow transplantation to peripheral stem cell transplantation Citation[21], Citation[22], Citation[26–28]. Also in this Scandinavian adjuvant high-dose chemotherapy study peripheral stem cells mobilized by leukocyte growth factors were used as a general treatment strategy. On the other hand over half of the direct health care costs in group A were attributable to the use of filgrastim.
A combination of cytotoxic drugs and leukocyte growth factors (granulocyte colony-stimulating factor, G-CSF; granulocyte macrophage colony-stimulating factor, GM-CSF) is the most effective treatment for the mobilization of stem cells Citation[26]. The use of growth factors both for mobilization and after re-infusion of the stem cells Citation[22], Citation[28–30] has been shown to reduce treatment costs: the hospital stay is shorter and the need for blood products and antibiotics is reduced. High concentrations of CD34+ cells in the graft have also been shown to reduce treatment costs Citation[22], which emphasizes the role of effective mobilization. The amount of CD34+ cells required in this trial was over 2 million cells/kg, which was the case in every patients treated in group B.
Stem cell transplantation is an expensive form of treatment. Its efficacy has been established in several malignancies, especially in the treatment of lymphomas Citation[30], while in the treatment of early and metastatic breast cancer it has not produced a superior outcome. Recently, however, growth factor-supported chemotherapy regimens given with a dose-dense strategy have resulted in overall survival gain Citation[31].
According to the present prospective study the mean direct health care costs of the adjuvant treatment were significantly higher in the HDCT + PSCT group (group B) than the tailored and dose escalated FEC with growth factor support (group A). The main reason for this was the higher number of hospital days in group B, which is in accord with studies in lymphomas treated with high-dose chemotherapy with stem cell support. In contrast to the costs of chemotherapy, those incurred by radiotherapy were closely similar in both groups, accounting only for 5% of total treatment costs at the oncology unit. Radiotherapy has been shown to be cost-effective in the curative and palliative treatment of several malignancies Citation[4]. In addition, radiotherapy delivered with modern techniques has been shown to reduce local recurrences and to improve overall survival Citation[23].
Treatment B produced more survival days than treatment A within the 5-year follow-up period, but the difference was not statistically significant. Sensitivity analysis confirmed this conclusion (the 95% CI of the difference included zero). However, sensitivity analysis also suggested that treatment A would be a strongly dominant alternative (less costly and more effective) in 84% of cases. Considering productivity costs as well reduces this percentage to 74, but in only 2% would treatment B be strongly dominant. Given the controversial nature of measuring and valuing productivity costs more emphasis should be placed on results based on direct health care costs. Also the cost-effectiveness acceptability curve for treatment B indicates that at any reasonable level of willingness to pay for an extra survival day, the probability of treatment B being acceptable is low.
Cost analysis and pharmacoeconomics have proved to be very important tools in analyzing the cost-effectiveness and efficiency of oncological interventions Citation[31–36]. According to a recent survey of the cancer costs in Europe the direct costs for cancer from the total health care costs varied from 6.5 to 7% (from 587 to 1 253 million €) in Nordic countries Citation[37]. In Sweden in year 2002 the inpatients hospital care dominated being 74% of the cancer health care costs, while the cancer drug costs at the same time counted only 10% of the costs Citation[37]. In addition the lost working years in Germany due to breast cancer were 65 years in year 2002. Based on all these cost analyses it is very important to have effective outpatient adjuvant breast cancer treatments. In addition in a recent study by Kievit et al. Citation[38] the new guidelines of the adjuvant treatment of breast cancer have affected the number of patients eligible to adjuvant studies and the cost-effectiveness ratio of about 4837 € per life year gained is well within the range of values that are generally considered acceptable. In our study HDCT with PSCT (group B) was significantly more costly than dose-escalated FEC with growth factor support (group A), when direct health care costs are considered, even though over half of the costs in group A comprised of costs of growth factors. In a majority of cases the tailored and dose-escalated FEC seems to be less costly and more effective than HDCT with PSCT as adjuvant treatment of breast cancer.
References
- Bergh J, Wiklund T, Erikstein B, Lidbrink E, Lindman H, Malmstrom P, et al. Marrow supported high dose therapy is not superior compared with tailored FEC in the adjuvant setting to high risk breast cancer. Results of a randomized study. Lancet 2000; 356: 1384–91
- Chang S, Long SR, Kutikova L, Bowman L, Finley D, Crown WH, et al. Estimating the cost of cancer: Results on basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999–2000. J Clin Oncol 2004; 22: 3524–30
- Radice D, Redaelli A. Breast cancer management. Quality of life and cost considerations. Pharmacoenonomics 2003; 21: 383–96
- Dunscombe P, Samant R, Roberts G. A cost-outcome analysis of adjuvant postmastectomy locoregional radiotherapy in high-risk postmenopausal breast cancer patients. Int J Radiat Oncol Biol Phys 2000; 48: 977–82
- Brown RE, Hutton J, Burrell A. Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 2001; 19: 1091–102
- Higa GM. Aromatase inhibitors for breast cancer: Pharmacoeconomic considerations. Expert Opin Pharmacother 2001; 2: 987–95
- Pagano E, Ponti A, Gelormino E, Merletti F, Mano MP. An economic evaluation of the optimal workload in treating surgical patients in a breast unit. Eur J Cancer 2003; 39: 748–54
- Verma S, Ilersich AL. Population-based pharmacoeconomic model for adopting capecitabine/docetaxel combination treatment for anthracycline-pretreated metastatic breast cancer. Oncologist 2003; 8: 232–40
- Mello MM, Brennan TA. The controversy over high-dose chemotherapy with autologous bone marrow transplant for breast cancer. Health Aff (Millwood) 2001; 20: 101–17
- Schulman KA, Stadtmauer EA, Reed SD, Glick HA, Goldstein LJ, Pines JM, et al. Economic analysis of conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoetic stem-cell transplantation for metastatic breast cancer. Bone Marrow Trans 2003; 31: 205–10
- Rodenhuis S, Richel D, van der Wall E, Schornagel JH, Baars JW, Koning CC, et al. Randomised trial of high-dose chemotherapy and haematopoetic progenitor-cell support in operable breast cancer with extensive axillary lymph-node involvement. Lancet 2003; 352: 515–21
- Rodenhius S, Bontenbal M, Beex LV, Wagstaff J, Ricehl DJ, Nooij MA, et al. High dose chemotherapy with hematopoietic rescue for high risk breast cancer. New Eng J Med 2003; 349: 7–16
- Hortobagyi GN, Buzdar AU, Theriault RL, Valero V, Frey D, Booser DJ, et al. Randomised trial of high-dose chemotherapy and blood cell autografts for high-risk primary breast cancer. J Natl Cancer Inst 2000; 92: 225–33
- Tallman MS, Gray R, Robert NJ, LeMaistre CF, Osborne CK, Vaughan WP, et al. Conventional adjuvant chemotherapy with or without high dose chemotherapy and autologous stem-cell transplantation in high-risk breast cancer. N Eng J Med 2003; 349: 17–26
- Elfenbein GJ. Stem cell transplantation for high risk-breast cancer. N Eng J Med 2003; 349: 80–2
- Wheatley K, Gray RG, Ives NJ. Correspondence. High dose chemotherapy for breast cancer. N Eng J Med 2003; 349: 1476–7
- Bergh J, Wiklund T, Erikstein B, Fornander T, Bengtsson N-O, Malmström P, et al. Dosage of adjuvant G-CSF (Filgrastim) supported FEC polychemotherapy based on equivalent hematological toxicity to high risk breast cancer. Ann Oncol 1998; 9: 1–9
- Ithaka R, Gentleman R. A language for data analysis and graphics. J Comput Graph Stat 1996; 5: 299–314
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet 2005;365:1665–717.
- Bergh J. Where next with stem-cell–supported high-dose therapy for breast cancer?. Lancet 2000; 355: 944–45
- Champlin RE. Peripheral blood progenitor cells: A replacement for marrow transplantation?. Semin Oncol 1996; 23: 15–21
- Brice P, Gordin S, Libert O, Marolleau JP, Makki Jextra JM, Extra JM, et al. Influence of the granulocyte growth factor on the cost of bone marrow autografts in oncologic hematology. Presse Medicale 1994; 23: 1512–5
- Overgaard M. Radiotherapy as part of multidisciplinary treatment strategy in early breast cancer. Eur J Cancer 2001; 37: 33–43
- Faulkner LB, Tucci F, Tamburini A, Tintori V, Lippi AA, Bambi F, et al. G. G-CSF serum pharmacokinetics during peripheral blood progenitor cell mobilization: Neutrophil count-adjusted dosage might potentially improve mobilization and be more cost-effective. Bone Marrow Trans 1998; 21: 1091–5
- Kath R, Hartmann M, Hoffken K. Pharmacoeconomic evaluation of high-dose chemotherapy and peripheral blood stem cell support in high-risk or poor-prognosis malignancies. J Cancer Res Clin Oncol 1998; 124: 288–90
- Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol 1995; 13: 2547–55
- Frey P, Stinson T, Siston A, Knight SJ, Ferdman E, Traynor A, et al. Lack of caregivers limits use of outpatient hematopoetic stem cell transplant program. Bone Marrow Trans 2002; 30: 741–8
- Bredeson C, Malcolm J, Davis M, Bence-Bruckler I, Kearns B, Huebsch L. Cost analysis of the introduction of PBPC for autologous transplantation: Effect of switching from bone marrow (BM) to peripheral blood progenitor cells (PBPC). Bone Marrow Trans 1997; 20: 889–96
- Meisenberg BR, Ferran K, Hollenbach K, Brehm T, Jollon J, Piro LD. Reduced charges and costs associated with outpatient autologous stem cell transplantation. Bone Marrow Trans 1998; 21: 927–32
- Meisenberg BR, Miller WE, McMillan R, Callgahn M, Sloan C, Brehm T, et al. Outpatient high-dose chemotherapy with autologous stem cell rescue for hematologic and nonhematologic malignancies. J Clin Oncol 1997; 15: 11–7
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradisher WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of intergroup trial C9741/Cancer and Leukemia Group B trial 9741. J Clin Oncol 2003; 21: 14311–439
- Peters WP, Rosner GL, Vredenburgh JJ, Shpall EJ, Crump M, Richardson PG, et al. Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: A report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol. 2005; 23: 2191–200
- Beltz SE, Yee GC. Pharmacoeconomics of cancer therapy. Cancer Control 1998; 5: 415–24
- Chirikos TN, Ruckdeschel JC, Krischer JP. Economic analysis of cancer treatment costs: Another tool for oncology managers. J Oncol Manag 2001; 10: 11–7
- Koopmanschap MA, Touw KC, Rutter FF. Analysis of costs and cost-effectiveness in multinational trials. Health Policy 2001; 58: 175–86
- Ridenour TA, Treloar JH, Dean RS. Utility analysis for clinical decision-making in small treatments settings. Int J Neuroscience 2003; 113: 417–30
- Wilking N, Jönsson B. A pan-European comparison of regarding patient access to cancer drugs. Karolinska Institute, StockholmSweden 2005
- Kievit W, Bolter MJ, van der Wilt GJ, Bult P, Thunissen FB, Meijer J, et al. Cost-effectiveness of new guidelines for adjuvant systemic therapy for breast cancer. Ann Oncol 2005; 16: 1874–81