Abstract
The Stockholm Breast Cancer Study Group conducted a randomized trial during 1976 through 1990 comparing adjuvant tamoxifen versus control. A total of 2 738 postmenopausal women with invasive, early stage disease were randomised between tamoxifen for 2 or 5 years, or no adjuvant endocrine therapy. Among high-risk patients the treatment was given against a background of either postoperative, locoregional radiation or CMF-type chemotherapy. The median follow up was 18 years (range 11–25 years). There was a statistically significant (p =0.001) interaction between ER status and tamoxifen with no treatment benefit among receptor negatives. PgR-status had little additional predictive value. Among ER-positive patients tamoxifen reduced locoregional recurrences by 48%, contralateral breast cancers by 54%, distant metastases by 28%, and all events by 24% (p <0.001). On the other hand, there was a substantial increase of endometrial cancer associated with tamoxifen. There was no effect of tamoxifen on intercurrent mortality whereas breast cancer deaths were reduced by 31% (p <0.001) and overall mortality by 15% (p =0.01). Tamoxifen produced long-term benefits among estrogen receptor positive patients in terms of breast cancer-related events, but also an increased incidence of endometrial cancer. Despite long-term follow-up we observed no benefit with tamoxifen in terms of cardiovascular mortality.
In the mid 1970s the Stockholm Breast Cancer Study Group initiated a comprehensive program for the diagnosis, treatment and follow-up of women with breast cancer in the Stockholm area. The program, which is still on-going, includes clinical practice guide-lines and entails an extensive multidisciplinary collaboration between all those involved in breast cancer care. The purpose is to ensure a good and equal care for all patients in the region irrespective of, for instance, domicile or socioeconomic status. Only a few years after its initiation, the program covered about 90% of all new breast cancer cases in the area. Within the context of the program a controlled trial of adjuvant tamoxifen among postmenopausal women was initiated in 1976. Tamoxifen was introduced in the early 1970s for the treatment of advanced breast cancer. In the mid 1970s several international research groups considered it appropriate to evaluate the potential of the drug in the adjuvant setting.
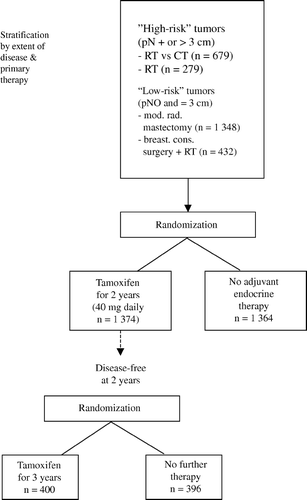
The Stockholm trial included both “low- risk”, node-negative and “high-risk” (typically node-positive) patients in order to evaluate the effect of adjuvant tamoxifen in a wide variety of patients with primary, operable disease. Among the high-risk patients, a 2×2 factorial study design was used which permitted the tamoxifen comparison being conducted concurrently with a comparison of postoperative radiation therapy versus adjuvant chemotherapy Citation[1].
The trial only included postmenopausal patients because in the mid 1970s the effect of the drug in premenopausal patients was not considered well established. There was no selection to the trial on the basis of hormone receptor status since its treatment predictive value in the adjuvant setting at the time was considered uncertain. However, prospectively collected data on receptor status were available in about 85% of the patients included in the trial.
The study was open for patient entry until 1990. At that time, there were convincing data from the overview of adjuvant tamoxifen trials showing a clinically worthwhile survival benefit with tamoxifen Citation[2]. Due to these results the randomization was considered unethical and the trial was closed for patient entry. Preliminary results of the trial were reported previously Citation[3–7]. However, this is the first report including all 2 738 randomized patients. The median follow-up was 18 years (range: 11–25 years).
Material and methods
The trial design was described in detail previously Citation[3–7]. In summary, tamoxifen (TAM) given postoperatively at a dose of 40 mg daily for 2 or 5 years was compared to no adjuvant endocrine therapy among postmenopausal women with a histologically verified invasive, unilateral breast cancer. A woman was considered postmenopausal if more than 6 months had elapsed since her last menstrual period. After a hysterectomy a woman was considered postmenopausal if aged above 50 years. The upper age limit was 70 years. Reasons for exclusion from the trial was inoperable local disease, distant metastases at the time of primary diagnosis, other concurrent cancers, medical contraindications to the treatment, and surgery which deviated from that stipulated in the protocol.
The design of the trial is summarised in . Prior to randomization the patients were stratified into four groups according to tumor stage, type of surgery and concomitant therapy: 1) “low risk” patients (defined as those with a node negative tumour and a tumor diameter-measured on the surgical specimen – less than 30 mm) treated with a modified radical mastectomy, 2) “low risk” patients treated with breast conserving surgery plus radiation therapy to the breast parenchyma (50 Gy/5 weeks), 3) “high risk” patients (defined as those with a node positive tumour or a tumor diameter-measured on the surgical specimen – more than 30 mm) treated with a modified radical mastectomy and randomly allocated between locoregional radiation therapy (RT, 46 Gy/4½ weeks) and adjuvant chemotherapy (CT, this group of patients constituted a separate stratum in a concurrent randomized trial of postoperative radiation therapy versus adjuvant chemotherapy), and 4) “high risk” patients treated with a modified radical mastectomy followed by RT (these were typically patients unwilling to participate in the mentioned concurrent randomized trial of RT versus CT or patients considered unfit to receive CT).
Randomization was by telephone to a central office where the patients’ identifiers were recorded before the allocated treatment was revealed to the responsible physician. Randomization was done using balanced lists prepared with a random number table according to a permuted block technique. The patients were stratified according to treatment centre and tumor stage, type of surgery, and concomitant treatment as previously mentioned. In each stratum the patients were randomly allocated between adjuvant TAM and no adjuvant endocrine therapy. This implied that the “low risk” patients were allocated between TAM and no adjuvant systemic treatment. The “high-risk” patients were randomized between TAM and no adjuvant endocrine therapy against a background of either CT or RT. No patient for whom a treatment was allocated was subsequently withdrawn from the analysis.
The treatment predictive ability of hormone receptor determinations in the adjuvant setting was considered uncertain in the mid 1970s. Therefore, there was no selection of patients to the trial on the basis of receptor content. However, prospectively collected data on estrogen receptor (ER) and progesterone receptor (PgR) content were available in 2 339 (85%) and 1 897 (69%) patients, respectively. All assays were done in one laboratory. Isoelectric focusing (IF) as previously described was used for patients included in the trial before 1988 Citation[8]. In 1988, the IF technique was replaced by quantitative enzyme-immunoassays Citation[9], Citation[10]. The receptor values were normalized to DNA content as measured by Burton Citation[11].
Patients with data on estrogen receptor content had slightly larger tumors and were somewhat more frequently node positive than the patients without such data but there was only a marginal difference in regard to age between these two groups (data not shown). There were no substantial differences between the patients with and without data on progesterone receptor content in regard to age, tumor size, or nodal status (data not shown).
A cut-off level of 0.05 fmol/microgram DNA was used to reproducibly distinguish receptor positive patients from receptor-poor/receptor-negative patients. We also investigated the treatment-predictive ability of other cut-off values.
Owing to a temporary shortage of radiation treatment capacity in the Stockholm area the concurrent randomization between radiation therapy and chemotherapy was deliberately unbalanced during March 1982 through May 1985: 2/3 of the patients were randomized to chemotherapy and 1/3 to radiation therapy. This explains the unbalanced number of patients included in the RT and CT groups. In total, 462 patients were allocated to the RT groups and 558 to the CT groups.
RT was initiated within 4–6 weeks of surgery. It was given with high-voltage technique. For “high-risk” patients the dose was 46 Gy with 2 Gy per fraction 5 days a week for a total treatment time of about 4½ weeks. The target volume included the chest wall, axilla, supraclavicular fossa and the ipsilateral internal mammary nodes. The treatment was individually planned in all cases. Dose planning of fields covering the chest wall and internal mammary nodes was 2-dimensional and based on one cross section at the mamillary plane. The chest wall was typically irradiated with 7–14 MeV electrons and the lymph node areas with Co-60 or 4–6 MV photons. Radiation therapy to the breast was an integral part of breast conserving surgery among “low-risk” patients (in practice, breast conservation was not a treatment option for “high-risk” patients at the time of the trial). The dose was 50 Gy with 2 Gy per fraction 5 days a week for a total treatment time of about 5 weeks. The target volume included the breast parenchyma. The treatment was individually planned in all cases. Dose planning was 2-dimensional and based on one cross section at the mamillary plane. The treatment was given with Co-60 or 4–6 MV photons using a standard tangential treatment technique.
For “high-risk” patients participating in the RT versus CT randomisation the chemotherapy protocol after 1978 was the same as in the first Milan trial Citation[12], that is, 12 courses of CMF (cyclophosphamide 100 mg/m2 orally on day 1–14, methotrexate 40 mg/m2 i.v. on day 1 and 8, 5-fluorouracil 600 mg/m2 i.v. on day 1 and 8). However, during the first 18 months of the study cyclophosphamide was replaced by chlorambucil 10–15 mg orally on day 1–8 and up to 18 months was allowed for the 12 courses to avoid dose reductions. Because of prolonged thrombocytopenia in some patients, the regimen was changed to the mentioned CMF regimen in 1978 and the cycle length was shortened to 28 days. Dose reductions were scheduled in case of hematological or gastrointestinal toxicity. Despite such reductions it was difficult to administer the chemotherapy to older women owing to drug toxicity. Therefore, the upper age limit in the CT versus RT trial was lowered to 65 years in 1980. In 1988, the protocol was amended to include six courses of chemotherapy instead of 12 since the overview of adjuvant chemotherapy trials did not indicate any advantage with regimens of longer versus shorter durations.
TAM (40 mg daily) was initiated within 2–4 weeks of surgery. The drug was thus administered concurrently with either radiation or chemotherapy. The protocol duration of treatment was initially 2 years. However, a new trial was initiated in 1983: TAM patients who were disease-free at 2 years were randomly allocated to 1) discontinue TAM, or 2) to continue for 3 years, that is, a total treatment period of 5 years. The details and results of this trial were reported previously Citation[13]. As some TAM patients were allocated to 2 years and some to 5 years of treatment, the average protocol duration of therapy was 2.9 years. About 4% of the patients allocated to tamoxifen did not start such therapy. The most common reasons were administrative error or patient refusal.
During 1976–1990 a total of 2 738 patients entered the trial of whom 1780 (65%) had “low-risk” tumours. The original protocol did not include a target sample size. Instead, the trial was open until 1990, that is, until information was available from the Oxford overview of adjuvant trials unequivocally showing a clinically worthwhile survival benefit with adjuvant TAM Citation[2]. At that time, it was considered unethical to withhold adjuvant TAM and the trial was closed for entry.
Patients who had been diagnosed less than one year before the trial was closed and who had been allocated to the untreated control group were informed about the potential benefits of adjuvant tamoxifen and were given the option to start such therapy on an individual basis. In total c. 1% of the patients allocated to the control group received adjuvant tamoxifen, either because of an administrative error, patient preferences, or because they were offered such therapy after the trial was closed for entry.
The trial protocol was approved by the Karolinska Institute's Research Ethics Committee.
Follow-up visits took place once every 3 months during the first 2 years, every 6 months during 2–5 years and yearly thereafter. Routine visits included a physical examination and an annual mammogram. Chest x-rays, bone scans, blood-tests, biopsies etc. were only done if clinical signs or symptoms indicated a possible relapse. The treatment after disease recurrence was decided individually for each patient by the responsible clinician.
The vital status of all patients was checked against regional population registers and the Swedish Cause of Death Registry. All patients were also checked against the Swedish Cancer Registry. The common end-date for follow-up was January 1, 2002. The median follow-up was 18 years (range: 11–25 years). A total of 20 patients (0.7%) were lost to follow-up due to emigration.
Overall and event-free survival (EFS) was estimated according to the Kaplan-Meier technique Citation[14]. The end-point in calculations of EFS was any event, that is, disease recurrence, contralateral breast cancer, other cancer, or death without a reported recurrence. Loco-regional recurrence was defined as a relapse on the chest wall or in the ipsilateral regional nodes. This implied that supraclavicular recurrences were recorded as loco-regional. Patients with synchronous loco-regional and distant recurrence were considered to have had distant recurrence as their first event. The information concerning second cancer as a first event was primarily based on information supplied by the responsible clinician to the trial centre. However, in these cases the clinical information was supplemented with data from the Swedish Cancer Registry on tumor site and histopathology. The trial protocol did not specify the clinical work-up needed to distinguish a new primary malignancy from a distant metastasis from the patient's primary breast cancer. This was left to the discretion of the responsible physician.
Crude cumulative incidence rates, that is, the failure probability for a particular type of event in the presence of other events, were estimated using the Kaplan-Meier technique generalized to include competing risks Citation[15]. Survival time distributions were compared with the log-rank test Citation[16]. Hazard rate ratios (relative hazard, RH) and 95% confidence intervals (95% C. I.) were estimated using Cox's proportional hazards model Citation[17]. Tests of interactions between treatment and various covariates were done by inclusion of product terms in the models.
Deaths among patients with a reported locoregional and/or distant recurrence were recorded as due to breast cancer. The cause of death among other deceased patients was recorded as the underlying cause of death reported by the Swedish Cause of Death Registry.
All randomized patients were included in the analyses irrespective of eligibility or exclusion criteria. The analyses were on the basis of intention to treat. No analyses were done on the basis of treatment received.
Preliminary results of the trial have been published previously Citation[3–7]. However, this is the first publication including all 2 738 randomized patients
Results
The TAM and control group were well balanced in regard to clinical characteristics. This held true both for the high-risk and the low-risk stratum ().
Table I. Distribution of patient characteristics by risk strata and allocated treatment
All patients
Patients allocated to TAM showed statistically significant reductions of locoregional recurrences, distant metastases, and contralateral breast cancers, but also an increase of second cancers, resulting in a net overall relative reduction of events with 16% (p < 0.001, ). There was a reduction of deaths due to breast cancer among the TAM patients corresponding to a relative reduction of 24% (p < 0.001), but also an 18% increase of deaths due to intercurrent causes which was of borderline statistical significance (p = 0.07). There was no beneficial effect of TAM on cardiovascular mortality (RH: 1.07) although the 95% confidence interval for this estimate was relatively wide. Overall mortality was reduced by 10% (p = 0.06) ().
Table II. Analysis of first events and cause specific mortality among all patients
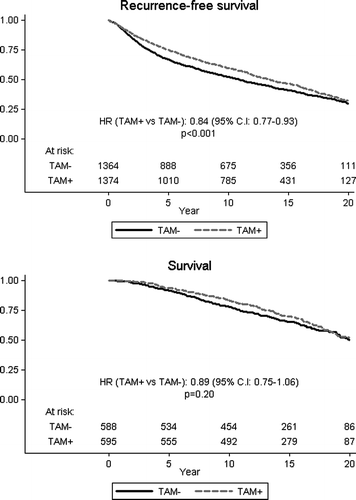
An analysis of events by period of follow-up () showed that disease recurrences and total deaths were reduced among the TAM patients mainly during the first 10 years whereas the relative hazards thereafter were close to or above unity. Breast cancer deaths, on the other hand, appeared to be reduced also during 10–14 years as well as 15–19 years although the confidence intervals for the relative hazards were wide and included unity. Cumulative event-free and overall survival are displayed graphically in .
Figure 2. Overall and event-free survival among all patients according to allocated treatment. Relative hazard (HR) and logrank p-values are indicated.
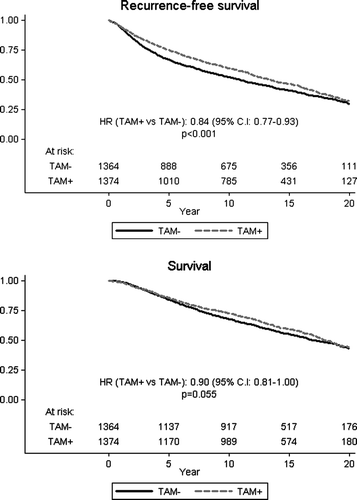
Table III. Analysis of events by period of follow-up
Treatment benefit versus hormone receptor status
There was a statistically significant interaction (p = 0.001) between the effect of TAM and ER-status (). A reduction of events associated with TAM was only observed among those classified as ER positive (relative hazard, RH: 0.77, p < 0.001), whereas no such treatment benefit was observed among the ER-negatives (RH: 1.18, p = 0.16).
Table IV. Analyses of the interaction between ER-content and the effect of adjuvant tamoxifen
A more detailed analysis of treatment benefit versus ER levels revealed that there was no such benefit irrespective of whether there had been no detectable levels of ER in the primary tumor, or if low levels (0.01–0.04 fmol/microg DNA) had been detected. Among patients with an ER content of ≥ 0.05 fmol/microg DNA, the benefit with TAM appeared unrelated to the ER concentration ().
The PgR-status of the primary tumor did not seem to modify the effect of TAM: the estimated relative reduction of events was only marginally greater among those classified as PgR-positive compared to the PgR-negatives (). The choice of different cut-off levels for PgR-positivity did not change this conclusion (data not shown). We observed no treatment benefit among those classified as ER-/PgR+ (RH:1.31), but as there were few patients and events in this subgroup, the confidence interval was wide.
Table V. Effect of tamoxifen by ER and PgR-status according to assay technique
In 1988 the receptor laboratory changed assay technique from isoelectric focusing (IF) to quantitative enzyme immuno assays (EIA). As the latter are generally regarded as being more accurate, we examined the treatment predictive ability separately for patients assayed with the two techniques (). The EIA techniques appeared to be more sensitive because fewer patients than previously were classified as receptor negative. This held true particularly for PgR-status: the proportion classified as PgR negative was 38% among those assayed with EIA compared to 56% among those assayed with IF. The corresponding proportions for ER status were 15% and 23%. The treatment benefit with TAM appeared to be marginally greater among those classified as ER positive with the EIA technique (RH: 0.71) compared to those classified with IF (RH: 0.76). Irrespective of assay technique, PgR status did not seem to modify the effect of TAM among the ER-positives. In fact, with the EIA techniques the treatment benefit was estimated to be larger among those classified as ER + /PgR- (RH: 0.43) than for the ER + /PgR+ subgroup (RH: 0.87), but due to small numbers these estimates were associated with wide confidence intervals. With the EIA techniques, only few patients (0.9%) were classified as ER-/PgR+ so a stable estimate of treatment effect for this subgroup was not possible due to small numbers.
Estrogen receptor positive patients
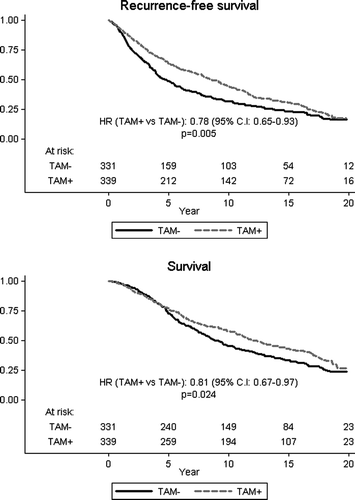
Given the qualitative interaction between ER status and treatment with no apparent benefit from TAM among those classified as receptor negative, we considered it appropriate to focus on treatment effects among ER positive patients (). In that subset, TAM reduced locoregional recurrences by 48%, contralateral breast cancers by 54%, distant metastases by 28%, and all events by 24%. On the other hand, there was a 59% increase of second cancers as first events. Breast cancer deaths were reduced by 31% (p < 0.001) and overall mortality by 15% (p = 0.01). As in the overall analysis, no statistically significant effect on intercurrent mortality was observed.
Table VI. Analysis of first events and cause specific mortality among ER positive patients
Comparisons of the treatment effect among the low and high risk subsets revealed slight variations in the estimated relative hazards for the different end-points, but no substantial differences (). These data are displayed graphically in and as cumulative event-free and overall survival.
2 versus 5 years of TAM
The randomized comparison of 2 versus 5 years of TAM showed a slightly lower overall event rate and fewer deaths with the longer treatment duration, but these differences were far from statistical significance (). As it might be more appropriate to compare a specific treatment duration with no treatment, we compared the outcome for patients included in the 2 versus 5 year trial with concurrent patients allocated to no TAM as part of the original trial provided they were event-free at 2 years after randomisation (). This analysis revealed that the patients allocated to 5 years of TAM had statistically significant fewer events (RH: 0.81) and breast cancer deaths (0.65) than those allocated to no TAM. The overall survival benefit in this comparison (RH: 0.80) was of borderline statistical significance (p = 0.07).
Table VII. Analysis of first events and cause specific mortality among patients randomly allocated to 2 versus 5 years of tamoxifen
Table VIII. Analysis of first events and cause specific mortality among patients randomly allocated to 2 or 5 years of tamoxifen, and concurrent patients allocated to no adjuvant endocrine therapy
Second cancers
Among patients allocated to TAM, a total of 164 (12%) were recorded with another cancer (other than contralateral breast cancer) as a first event compared to 94 (7%) among those allocated to no TAM (RH: 1.53, p < 0.001) (). Most of this excess concerned endometrial cancers and second lung cancers (). The excess of lung cancer typically concerned adenocarcinomas and tumors diagnosed during the first 10 years of follow-up.
Table IX. Number of new cancers (other than contralateral breast cancer) diagnosed as first events by allocated treatment
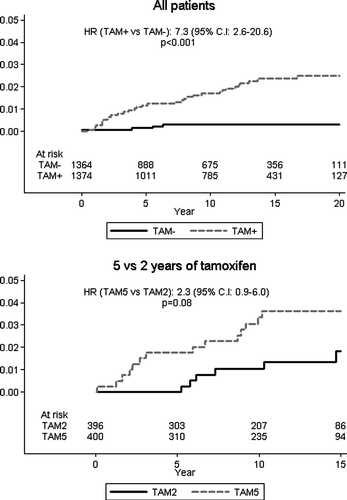
The cumulative incidence of endometrial cancer according to allocated treatment is displayed graphically in . In the overall analysis, the curves tended to diverge during the entire follow-up period, that is, even several years after cessation of active treatment. In the 2 versus 5 year comparison there was a statistically non-significant trend towards a higher cumulative risk among those allocated to 5 years (p = 0.08).
Figure 5. Cumulative incidence of endometrial cancer according to allocated treatment among all patients (left panel), and among those included in the randomized comparison of 2 versus 5 years of tamoxifen (right panel). Note that in the latter trial, the starting point for the curves is at 2 years after the initial entry into the trial. Number of patients at risk, relative hazards, and logrank p-values are indicated.
Discussion
Adjuvant TAM has for many years been a cornerstone of the postoperative management of patients with early stage breast cancer with hormone receptor positive disease. Early results from controlled trials initiated during the 1970s which became available during the early to mid 1980s laid the foundation for this treatment policy Citation[3], Citation[18], Citation[19]. The Stockholm adjuvant TAM trial was one of those studies. In fact, the Stockholm trial is one of the world′s largest studies of adjuvant systemic therapy versus an untreated control group. Besides being a large trial, another strength of the study was that inclusion was not based on hormone receptor status although prospective data on receptor status, with all assays done in one laboratory, were available on 85% of the patients. This has permitted evaluations of the treatment predictive ability of estrogen and progesterone receptor levels. Other strengths of this report include the long follow up (median 18 years), the low number of patients lost to follow-up (<1%), and the possibility to check the vital status of the patients, second cancers, and the officially recorded cause of death through computerized linkages to population based Swedish registers.
Previously, we have reported early findings from the trial for subsets of patients Citation[3–7], second cancer incidence Citation[20], Citation[21], risk of intercurrent morbidity & cardiovascular disease Citation[22], Citation[23], effects on bone mineral density Citation[24], and survival after recurrence Citation[25]. However, this is the first report based on all patients entered in the trial between 1976 through 1990.
There was a qualitative interaction between the effect of TAM and the estrogen receptor levels in the primary tumor: no benefit was observed among patients whose tumors were classified as ER negative. Moreover, the 95% confidence interval for the relative hazard excluded but a marginal treatment benefit in this subgroup (). Among those classified as ER positive there was little correlation between the receptor levels and treatment benefit, that is, those with low levels appeared to derive roughly the same benefit as those with high levels. The benefit with TAM among the ER positives did not appear to be modified by PgR status: the estimated benefit was the same among those classified as ER + /PgR+ as in the ER + /PgR- subset.
All assays were done in one laboratory which in 1988 switched from techniques based on isoelectric focusing to more modern, antibody based techniques. The newer methods appeared to be more sensitive because the proportion of patients classified as receptor negative was smaller among those entered in the trial after 1988 than previously. The shift was most prominent for the PgR assay: the proportion classified as negative decreased from 56 to 38%. We observed a similar but much smaller shift with the ER assays: a decrease from 23 to 15% negatives. Although the antibody based techniques may have been more sensitive, their treatment predictive ability appeared only marginally better than the older techniques: the relative hazard (TAM versus control) for patients classified as ER positive was 0.76 with the older technique compared to 0.71 with the new technique. A lack of predictive ability with the PgR assay held true both with the old and the new technique. However, these are only indirect comparisons as they concern patients entered in the trial during different periods. Therefore, the results may be confounded by differences in patient characteristics and length of follow-up.
We found that, at a median follow-up of 18 years, among patients allocated to TAM (for a median duration of about 3 years) and whose primary tumors had been classified as estrogen receptor positive, breast cancer mortality was reduced by about one third and overall mortality by about one fifth (). As expected, the benefit in terms of overall mortality tended to diminish somewhat after 10 years as deaths from intercurrent causes become more frequent with time in an ageing population. However, we observed a benefit in terms of breast cancer mortality during at least 20 years. These observations confirm and extend the results of the Oxford overview Citation[26] showing a sustained survival benefit associated with TAM among receptor positive patients extending up to at least 15 years.
TAM therapy results in decreased serum cholesterol levels Citation[27]. It has therefore been hypothesized that long term adjuvant therapy with the drug might lead to a decrease in cardiovascular mortality. In fact, early findings from this trial showed a substantially reduced incidence of hospital admissions due to cardiac disease among the patients allocated to TAM, particularly among those allocated to 5 years of treatment Citation[23]. In this long term follow-up, we observed no decrease in cardiovascular mortality among the TAM patients. In particular, we observed no such decrease associated with the longer treatment duration in the 2 versus 5 year comparison. These results are in line with the latest results from the Oxford overview which also failed to demonstrate a beneficial effect of long-term TAM on cardiovascular mortality Citation[26]. There was a marginal reduction of deaths due to ischemic heart disease, but this was balanced by an increased risk of death from thrombembolic events resulting in no overall difference.
The 2 versus 5 year randomisation was part of a nationwide collaborative trial which has accrued a total of more than 4 000 patients. A preliminary analysis of that trial based on the first 3 500 patients showed that the patients allocated to 5 years of TAM had significantly fewer treatment failures and a significantly better overall survival than those treated for only 2 years Citation[28].
We observed a significantly increased risk of second cancer (other than contralateral breast cancer) as the first event among patients allocated to TAM (). Some of this excess may have been due to competing risks since recurrence-free survival was better for those allocated to TAM resulting in more event-free TAM patients being at risk. We reported previously an excess risk for various gastrointestinal sites Citation[21]. However, an increased risk of gastrointestinal cancer associated with TAM has not been confirmed by the Oxford overview Citation[26]. In this update we also observed an increased number of second lung cancers among the TAM patients. However, a more detailed analysis revealed that this excess mainly concerned adenocarcinomas and tumors diagnosed during the first 10 years of follow-up. These observations suggest that the excess may be explained by distant metastases from breast cancer having been incorrectly diagnosed as primary lung cancers. Moreover, an association between TAM and lung cancer was not suggested by the latest results from the Oxford overview Citation[26].
The Stockholm trial was the first adjuvant TAM trial to observe an association between long term TAM and an excess risk of endometrial cancer Citation[20]. Following our first report in 1989, several other TAM trials have confirmed our initial observation. In this update, we confirmed the previously reported excess of endometrial cancer (p < 0.001), and the trend towards an increased risk for those allocated to 5 years of treatment compared to those allocated to 2 years (p = 0.08).
The trial was supported by funds from the Stockholm Cancer Society. We are greatly indebted to Mrs Ulla Johansson for excellent data managing.
References
- Rutqvist, LE, Johansson, H. Long-term follow-up of the Stockholm randomized trials of postoperative radiation therapy versus adjuvant chemotherapy among ‘high risk’ pre- and postmenopausal breast cancer patients. Acta Oncol 2006; (in press)
- Early Breast Cancer Trialist′s Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31 000 recurrences and 24 000 deaths among 75 000 women. Lancet 1992;339:1–15, 71–85.
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Nordenskjöld B, Skoog L, et al. The Stockholm trial on adjuvant tamoxifen in early breast cancer. Correlation between estrogen receptor level and treatment effect. Breast Cancer Res Treat 1987; 10: 255–66
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Rotstein S, Skoog L, et al. Radiation therapy, chemotherapy and tamoxifen as adjuncts to surgery in early breast cancer: A summary of three randomized trials. Int J Radiation Oncol Biol Phys 1989; 16: 629–39
- Rutqvist LE, Cedermark B, Fornander T, Glas U, Johansson H, Nordenskjöld B, et al. The relationship between hormone receptor content and the effect of adjuvant tamoxifen in operable breast cancer. J Clin Oncol 1989; 7: 1474–84
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Rotstein S, Skoog L, et al. Randomized trial of adjuvant tamoxifen combined with postoperative radiation therapy or adjuvant chemotherapy in postmenopausal breast cancer. Cancer 1990; 66: 89–96
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Rotstein S, Skoog L, et al. Randomized trial of adjuvant tamoxifen in node negative postmenopausal breast cancer. Stockholm Breast Cancer Study Group. Acta Oncol 1992; 31: 265–70
- Wrange Ö, Nordenskjöld B, Silfverswärd C, Granberg P-O, Gustafsson J-Å. Isoelectric focusing of estradiol receptor protein from human mammary carcinoma. A comparison to sucrose gradient analysis. Eur J Cancer 1976; 12: 695–700
- Fernö M, Borg Å, Norgren A. A comparison of two steroid receptor assays in breast cancer: Dextran-coated charcoal and isoelectric focusing. Anticancer Res 1983; 3: 243–6
- Fernö M, Borg Å, Sellberg G. Enzyme immuno assay of estrogen receptor (ER) in breast cancer biopsy samples. A comparison with isoelectric focusing and detection of ER during tamoxifen treatment. Acta Radiol Oncol 1986; 25: 171–5
- Burton KA. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of DNA. Biochem J 1956; 62: 315–22
- Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–10
- Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 1996; 88: 1543–9
- Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Marubini E, Valsecchi MG. Competing risks. Statistics in practice: Analysing survival data from clinical trials and observational studies, V Barnett. John Wiley and Sons, ChichesterUK 1995; 331–63
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–48
- Cox DR. Regression models and life tables. J Roy Statist Soc B 1972; 34: 187–220
- Baum M, Brinkley DM, Dossett JA, McPhersson K, Patterson JS, Rubens RD, et al. Improved survival among patients treated with adjuvant tamoxifen after mastectomy for early breast cancer. Lancet 1983; 2: 450
- Stewart HJ, Prescott R. Adjuvant tamoxifen therapy and receptor levels. Lancet 1985; 1: 573
- Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfverswärd C, et al. Adjuvant tamoxifen in early breast cancer: Occurrence of new primary cancers. Lancet 1989; 1: 117–20
- Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Skoog L, et al. Adjuvant tamoxifen in early-stage breast cancer: Effects on intercurrent morbidity and mortality. J Clin Oncol 1991; 9: 1740–8
- Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst ;;87 1995; 3: 645–51
- Rutqvist LE, Mattsson A. Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. The Stockholm Breast Cancer Study Group. J Natl Cancer Inst 1993; 85: 1398–406
- Fornander T, Rutqvist LE, Sjöberg HE, Blomqvist L, Mattsson A, Glas U. Long-term adjuvant tamoxifen in early breast cancer: Effect on bone mineral density in postmenopausal women. J Clin Oncol 1990; 8: 1019–24
- Fornander T, Rutqvist LE, Glas U. Response to tamoxifen and fluoxymesterone in a group of breast cancer patients with disease recurrence after cessation of adjuvant tamoxifen. Cancer Treat Rep 1987; 71: 685–8
- Early Breast Cancer Trialist′s Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005; 365: 1687–717
- Rössner S, Wallgren A. Serum lipoproteins and proteins after breast cancer surgery and effects of tamoxifen. Atherosclerosis 1984; 52: 339–46
- Nordenskjöld B, Rosell J, Rutqvist LE, Malmström PO, Bergh J, Bengtsson NO, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: Results from a randomized trial. J Natl Cancer Inst 2005; 97: 1609–10