To the Editor
Total body irradiation (TBI) has been widely utilized as part of the conditioning regimen for hematopoietic cell transplantation Citation[1]. However, with traditional TBI techniques the entire body is irradiated and non-involved organs such as the lungs, eyes, liver and kidneys receive potentially unnecessary irradiation Citation[2–5]. Multiple other techniques exist, but all are limited by their inability to shield non-involved organs Citation[6–8].
Relapse is a major obstacle to the success of bone marrow transplantation Citation[9–12]. One approach to reduce relapse would be to intensify the conditioning radiation or to direct it to sites of disease. Hawkins et al. showed the feasibility of total marrow irradiation in poor-risk Ewing sarcoma Citation[13]. Historically, some of these high risk patients relapse in bony sites distant from the primary tumor, presumably due to areas of micro-metastatic disease that were resistant to the conditioning chemotherapy. Although increasing the TBI dose may improve the relapse free survival, attempts at dose escalation have been associated with higher rates of severe toxicity Citation[9].
Intensity modulated radiotherapy (IMRT) allows precise focus of radiotherapy to the target as well as avoidance of non-target structures. Tomotherapy is a unique method to deliver IMRT and has the advantage that the beam travels helically along the axis of the patient. We have recently performed a feasibility study targeting bone marrow with image guided tomotherapy using an anthropomorphic phantom and demonstrated selective conformal irradiation to the bone marrow with a dose reduction to non-hematopoetic tissues, including the lungs, eyes, kidneys, and heart Citation[14]. As a continuation of that work, we present the first use of TMI treatment to the bone and bone marrow spaces along the entire axis of a patient using helical tomotherapy as part of bone marrow transplant conditioning regimen.
Methods
Peripheral blood progenitor cells were collected using leukophoresis following high dose chemotherapy as previously described Citation[10]. This product was then purged of residual malignant cells and cyropreserved as previously described Citation[11]. Following TMI, the patient received conditioning chemotherapy with busulfan (targeted, 4 mg/kg divided q 6 h on days −8 to −6), melphlan (50 mg/m2 on day −5 and −4) and thiotepa (250/m2 on days −3 and −2) as previously described by Fraser et al. Citation[10].
The patient was enrolled on this dose escalation trial to receive 600 cGy in 3 fractions. The patient was immobilized using a Vac-Lok™ bag to provide a consistent treatment position for initial CT scanning, pre-treatment MVCT imaging and for final treatment delivery. For tomotherapy treatment planning, a CT image set of the whole body was required. Since our CT scanner can scan a maximum length of 98 cm and the height of the patient (180 cm) exceeded that limit, two image sets (upper body and lower body) were scanned. To align these two scan sets, fiducial markers were placed in the sagital, coronal and transverse planes at the head, chest, abdomen, femur and ankle to provide consistent alignment of the patient.
Both image sets were exported to a Varian Eclipse planning station (Varian Medical Systems Inc., Palo Alto, California, USA) for contouring. The clinical target volume (CTV) included the entire skeletal system. For upper body, bone was contoured in four regions: (i) bone of the skull, (ii) thoracic bone, (iii) upper extremities, and (iv) pelvic bone. To account for set-up variability and breathing motion, a planning target volume (PTV) was generated with a 1 cm margin around CTV. The resulting images and contours were then transferred to the Tomotherapy HiArt Planning Station (Tomotherapy, Inc., Madison, Wisconsin, USA). A helical tomotherapy treatment plan was created from this data. The prescription dose was 200 cGy/fx (for 3 fractions) for a total dose of 600 cGy to the PTV. The normal tissue dose constraints utilized were based on the results of the survey of clinical outcome of target dose and dose limits to various organs at risk (OARs) such as lung, kidneys, eyes, liver, bowel, and bladder. For lower body TMI treatment, scrotum was completely blocked and dose to healthy tissues were minimized. Maximum importance was given to target dose coverage. The constraints on dose and penalty were adjusted accordingly during optimization. The field width, pitch, and modulation factor (MF) used for the treatment planning optimization were 5 cm, 0.3 and 1.8 respectively. The dose volume histograms (DVHs) were calculated for the target and individual OARs.
The delivery quality assurance (DQA) was evaluated with the use of: (a) direct ion chamber (Model A1SL ion chambers, Standard Imaging, Madison, USA) dose point measurements and (b) extended dose range (Kodak EDR) film dose profile measurements taken in a 30 cm diameter and 36 cm long cylindrical solid water phantom at selected locations critical to the treatment such as head and neck, abdomen, femur, junctions. The isorad cylindrical diodes (1.36 g/cm2 build up for 6 MV photons, effective detection area 1.65×1.65 mm2) (Sun Nuclear Corporation, Florida) were calibrated and used for in-vivo dosimetry study at different sites such chest, ankle, knee.
Prior to every treatment, sets of limited MVCT scans were taken in selected regions of the head, neck, chest, abdomen, femur and ankle using normal mode (4 mm slice thickness) of the body and fused with the treatment planning CT scan using bony anatomy and soft tissue images such as lungs. MVCT scans () were obtained. Image fusions were evaluated by the attending physician and physicist. Any translational shifts suggested by the image fusion results were applied to the final patient set-up prior to treatment delivery. Additional selected MVCT scans were performed after treatment to verify patient immobilization. Treatment, including the MVCTs, took approximate 80 min each day. The Translational shifts in pre-treatment and post-treatment are shown in .
Figure 1. MVCT scan taken at the abdomen before treatment delivery. MVCT scan set was fused with treatment planning CT scan. The transverse sectional views are shown in (A) Tomo MVCT image and in (B) reference kVCT image. The fusion column (C) presents the kVCT in gray scale with the MVCT superimposed with a level of transparency in yellow. Similarly, the sagital sectional views and the coronal sectional views are shown in D, E, F and G, H, I respectively.
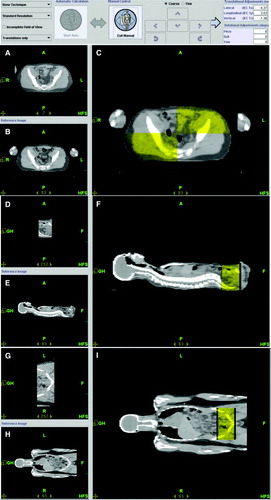
Table I. Translational (in IEC Tx, Ty, Tz) shift at various anatomical sites for pre-treatment and post-treatment using MVCT-kVCT fusion.
Results and discussion
The patient developed severe nausea and vomiting after the first fraction of Tomotherapy TMI. He was subsequently premedicatead with dexamethasone and ondansetron and had no further emesis or nausea. He showed neutrophil engraftment on day 11 and platelet engraftment by day 58. He is currently well at >150 days post transplant with no evidence of disease. Other than above there were no adverse effects of TMI and his overall treatment course was similar to others treated with this conditioning regimen.
The planned radiation conformed to all bone marrow sites. Dose volume histograms (DVHs) and isodose distributions to the target and OARs are shown in . The median dose to various organs are also tabulated () for 6Gy. The dose homogeneity index Citation[14] was 0.89, 0.92, 0.91 and 0.96 for thoracic bone, upper extremities, bone of skull and pelvic bone, respectively. The dose inhomogeneity was higher for thoracic bone due to dose restriction to lungs. We were able to reduce dose to lens, kidneys, and liver in addition to lung dose reduction compared to the standard lateral technique that we use in our clinic Citation[6]. The dose reduction to various OARs relative to conventional TBI varied from 30–88%.
Figure 2. Sectional view of dose painting: (I) transverse, (II) sagital, (III) coronal view for upper part of the body, and (IV) transverse, (V) sagital, (VI) coronal view for lower part of the body; (VII) The dose volume histogram for PTV(A), liver(B), heart(C), eye(D), lungs(E), spleen(F), left kidney(G), right kidney(H), lens(I), scrotum(J), and (V) DVH for bony anatomy: Upper body PTV (A), thoracic bone(A1); bone of skull(A2); pelvic bone(A3); right extremities(A4) are shown in lower middle panel (VIII). Right lower panel (IX) shows DVH for lower body PTV (A’).
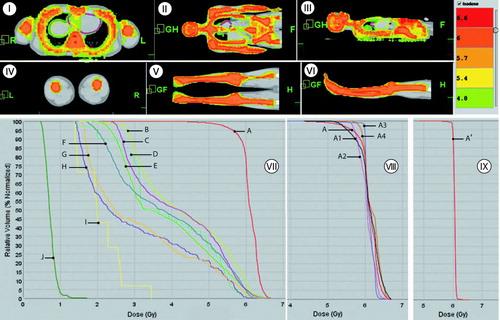
Table II. Median dose in various organs for prescription dose (Rx) of 6 Gy.
The dose delivery verification test (using ion chambers) on the phantom at different anatomical sites of the body resulted within ±3% (neck: 1.8%, femur:−2.1% and ankle: 2.4%) of the expected dose calculated from the treatment planning station. Isodose distribution comparisons between planned and delivered dose on EDR film at a single plane was within the tolerance of IMRT quality assurance. During treatment, in-vivo diode measurements at the chest and right knee were 224.3 cGy and 164.4 cGy compared to expected dose of 223±5 cGy, and 171±15 cGy respectively. Expected dose presented here includes the uncertainty due to the high dose gradient in the region and uncertainty in diode placement. Measured delivered dose was within a 5% of predicted dose from the treatment planning. Although the DQA test was essential for quality assurance of the treatment, in-situ diode measurement gave further confidence of the accuracy of the delivered dose.
External radiation, targeting all bone marrow sites has become clinically feasible. This trial was initiated based on our feasibility study using an anthropomorphic phantom Citation[14]. For clinical use, we added a 1 cm margin around the bone marrow to account for breathing motion and uncertainty in MVCT based patient localization. This novel bone marrow targeted therapy may allow for delivery of a radiation dose that is equivalent to TBI, but less toxic. Alternatively, it may also allow for radiation dose escalation of the marrow spaces, thereby enhancing the efficacy of radiation. Wong et al. Citation[15] recently included TMI in the conditioning therapy patients with acute myeloid leukemia (AML) undergoing hematopoietic cell transplantation. In their treatment modality, tomotherapy treatment was delivered for the upper part of the body and standard linac beam (anterior and posterior field) was used for the lower part of the body, with the common argument that the lower part of the body does not have critical organs. However, one may also favor reduction of radiation dose in any healthy tissue if possible.
The current report describes our clinical experience of TMI treatment targeting bone and bone marrow spaces along the entire axis to reduce radiation dose to healthy tissue including all the critical organs. This is part of a dose escalation trial to determine the maximum tolerated dose (MTD) of tomographic total marrow irradiation (TMI) when given prior to an alkylator-intensive conditioning regimen for the treatment of high risk or relapsed solid tumors. Our plan is to increase the dose in cohorts of three patients by 200 cGy per cohort. The protocol allows for post transplant Tomotherapy “selective boost” irradiation to sites of disease identified prior to high dose chemo- and irradiation therapy. Highly conformal and optimized dose-escalated radiation therapy along with high dose conditioning chemotherapy may result in synergistic tumor killing. Until now, this has not been possible due to the risk of unacceptably high regimen-related morbidity. Thus, the ideal solution would be to precisely deliver radiation to the sites of both primary disease and areas of occult micro-metastatic disease, while sparing areas that will likely not benefit from radiation. The TMI helical tomotherapy treatment required 16 min of beam-on time for the upper half of the body and 15 min for the lower half of the body.
The ability to verify the target regions before radiation delivery is particularly important for treatment of the entire marrow of the body. The 3-D MVCT scans of various sections of the body were extremely useful for pre-treatment patient setup. The limited MVCT image scan sets took 15 min for the upper half of the body and 10–15 min for the lower half of the body. Treatment delivery procedure for the upper half of the patient's body took 45 min. Total treatment time for the lower half of the body was approximately 35 min. Since the treatment was lengthy, we repeated the MVCT scan after the treatment to verify that the patient did not move during the long treatment procedure. Post treatment image scans indicated 5 mm movement () from pretreatment position in most situations. This is well within our PTV margin (10 mm). Confirmation of the relative position and shape of the target and organs at risk during daily treatment is essential for accurate conformal dose delivery. In future, real time tracking of patient positioning and reduction of MVCT scanning time may be necessary for TMI dose escalation study.
Conclusions
We show that helical tomotherapy targeting the bone marrow of the whole body is clinically feasible. The clinical implementation of intensity modulated radiation to conform the radiation dose to all sites of active bone marrow provides a new treatment modality for the pre-conditioning regimen for bone marrow transplant that is potentially less toxic and more efficacious. Our current report is a first step towards a series of clinical studies to determine whether TMI is a beneficial addition to the conditioning regimen of patients undergoing hematopoietic cell transplantation.
References
- Gilson D, Taylor RE. Total body irradiation. Br J Radiol 1997; 70: 1201–3
- Keane TJ, Van Dyk J, Rider W. Idiopathic interstitial pneumonia following bone marrow transplantation: The relationship with total body irradiation. Int J Radiat Oncol Biol Phys 1981; 7: 1365–70
- Belkacemi Y, Ozsahin M, Pene F, Rio B, Laporte JP, Leblond V, et al. Cataractogenesis after total body irradiation. Int J Radiat Oncol Biol Phys 1996; 35: 53–60
- Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys 1995; 31: 1249–56
- Murdych L., Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant 2001; 28: 283–7
- Dusenbery, KE, Gerbi, BG. Total body irradiation in conditioning regimens for bone marrow transplantation. Chapter 28; p 499–518. In:. Technological Basis of Radiation Therapy. SH Levitt, Potish, RA, Khan, FM, Perez, CA, editors. 3rd ed. Lippincott, Williams & Wilkins.
- Pla M, Chenery SG, Podgorsak EB. Total body irradiation with a sweeping beam. Int J Radiat Oncol Biol Phys 1983; 9: 83–9
- Hui SK, Douglas Henderson D, Das RK, Thomadsen B. CT based analysis of dose homogeneity in total body irradiation using lateral beam. J Appl Clin Med Phys 2004; 5: 1–9
- Clift RA, Buckner CD, Appelbaum FR, Bryant E, Bearman SI, Petersen FB, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: A randomized trial of two irradiation regimens. Blood 1991; 77: 1660–5
- Fraser CJ, Weigel BJ, Perentesis JP, Dusenbery KE, DeFor TE, Baker KS, et al. Autologous stem cell transplantation for high-risk Ewing's sarcoma and other pediatric solid tumors. Bone Marrow Transplant 2006; 37: 175–81
- Reynolds CP, Seeger RC, Vo DD, Black AT, Wells J, Ugelstad J. Model system for removing neuroblastoma cells from bone marrow using monoclonal antibodies and magnetic immunobeads. Cancer Res 1986; 46: 5882–6
- Anderson JE, Appelbaum FR, Schoch G, Barnett T, Chauncey TR, Flowers ME, et al. Relapse after allogeneic bone marrow transplantation for refractory anemia is increased by shielding lungs and liver during total body irradiation. Biol Blood Marrow Transplant 2001; 7: 163–70
- Hawkins D, Barnett T, Bensinger W, Gooley T, Sanders J. Busulfan, melphalan, and thiotepa with or without total marrow irradiation with hematopoietic stem cell rescue for poor-risk Ewing-Sarcoma-Family tumors. Med Pediatr Oncol 2000; 34: 328–37
- Hui SK, Kapatoes J, Fowler J, Henderson D, Olivera G, Manon RR, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys 2005; 32: 3214–24
- Wong JY, Liu A, Schultheiss T, Popplewell L, Stein A, Rosenthal J, et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: An alternative to standard total body irradiation. Biol Blood Marrow Transplant 2006; 12: 306–15
