Abstract
Arsenic trioxide (As2O3) has demonstrated effectiveness in treating acute promyelocytic leukemia (APL). Therefore the FDA has approved it to treat APL. In patients with refractory metastatic colorectal carcinoma (CRC), we assessed the efficacy and toxicity of As2O3/AA (ascorbic acid) as the outcome of this trial. Five patients with refractory metastatic CRC who failed all previous standard chemotherapy were enrolled in this study. They were treated with 0.25 mg/kg body weight/day As2O3 and 1000 mg/day of ascorbic acid for 5 days a week for 5 weeks. Each treatment cycle extended for 7 weeks with 5 weeks of treatment and 2 weeks of rest. All the patients developed moderate to severe toxic side effects to arsenic trioxide/AA therapy and therefore the study was discontinued. No CR (complete remission) or PR (partial remission) was observed. CT scans demonstrated stable or progressive disease. Three of the five patients died within 2 to 5 months after cessation of the therapy. None of the deaths could be related to this clinical trial. Two years of follow-up study showed that two patients were alive with stable disease. Under the current treatment regimen all patients developed moderate to severe side effects with no clinically measurable activity. As an alternate, efforts may be made to reduce the dose and arsenic trioxide may be combined with other standard regimen in reversing the chemo resistance.
To the Editor
A majority of colorectal cancer patients become refractory to standard chemotherapy. It results in treatment failure and eventual death. Therefore, identification of new agents with better anti tumor activity or ability to delay the onset or reverse resistance development remains the highest priority for clinical investigators in treating refractory tumor.
Recently arsenic trioxide (As2O3) has been demonstrated to have clinical efficacy over Acute Promyelocytic Leukemia (APL) Citation[1]. As a single agent it has induced complete remission (CR) with minimal side effects Citation[2]. In the USA, CR was observed in 80 to 90% of APL patients treated with As2O3Citation[3]. Based on these studies the US Food Drug Administration approved As2O3 for the treatment of APL.
Studies with human colon cancer cell lines, HT29 (5-FU sensitive), HT29FU (5-FU resistant developed from the sensitive variant) and SW480 (innately 5-FU resistant) showed that 1 µm concentration of As2O3 was effective in killing these cells. It also down regulated the expression of thymidylate synthase (TS) (Subbarayan et al., unpublished observations). One of the reasons proposed for the resistance to 5-FU is increased expression of TS in tumor cells Citation[4]. It is possible to sensitize the cells by reducing the expression of TS. Therefore, any agent that could down regulate TS has a potential to reverse the resistance to 5-FU.
Success of As2O3 in bringing about CR in APL patients, our in vitro experience on colon cancer cells HT29, HT29FU, SW480 and efficacy of As2O3/AA in other pre-clinical and clinical trials Citation[5] provided the rationale for this study. In this clinical trial we assessed the toxicity and efficacy of As2O3/AA in patients with refractory metastatic CRC. We also determined its effect on the expression of thymidylate synthase in vivo. Here we report the outcome of a phase II clinical trial using As2O3/AA therapy in patients with refractory metastatic colorectal carcinoma and its effect on the expression of TS mRNA in leukocytes.
Patient and methods
Patients with advanced refractory metastatic colon cancer were eligible for this study. Failure to respond to two different standard chemotherapy regimens was considered refractory. Patients must be 18 years and older. The clinical criteria included histologically confirmed stage IV colon cancer, demonstrated disease progression, bi-dimensionally measurable disease and life expectancy of more than two months. Patients should have ECOG performance status of 0–2. White blood cell count of >3 000 cells/mm3 and platelet count of >100 000 cells/mm3 were considered to be within acceptable limits. If patients’ initial WBC count is <3 000 but >1 000/mm3 and/or platelets are <100 000 but >50 000/mm3 secondary to documented bone marrow involvement, the patient will also be eligible for the study. Patients should have adequate renal function as documented by serum creatine, bilurubin level of less than 2 and SGOT level of less than four times the normal limit. Serum magnesium concentration of 1.4–2.0 mEq/L and potassium of 3.5 to 5.0 mMol/L was considered acceptable.
Patients should not have received any form of chemo- and/or radio- therapy for four weeks prior to the start of this study. The patients should be free of any toxic side effects from prior treatment. During the participation in this clinical trial the enrolled patients were excluded from receiving any other form of therapy.
Metastases of tumor to CNS, history of prior neurological disorders (grade 3 or higher by the NCI Common Toxicity Criteria, in particular seizure disorders) were also a reason for exclusion from this study. Pregnant women were also excluded and patients of childbearing age were advised against pregnancy. Prior to enrollment, all patients were counseled about investigational nature of the treatment and a written informed consent was obtained as per the institutional review board (IRB approval No. #01/576B) guidelines.
Treatment Scheme
Each patient was scheduled to receive an intravenous dose of 0.25 mg/kg/day As2O3 as Trisenox (Cell Therapeutics Inc, USA) injected over a period 1–4 hours daily, for 5 consecutive days of the week for 5 weeks. Within 30 min after the administration of As2O3, 1000 mg of ascorbic acid was administered intravenously over 15 to 30 min period. A two-week rest period was allowed between each cycle. Therefore, each treatment cycle consisted of 5 weeks of therapy and 2 weeks of rest. Three additional cycles of treatment at the same doses were permitted, if tolerated by the patient. Treatment can be discontinued due to 1) unacceptable toxicity, 2) no clinical benefit or 3) at patient's request. No other chemotherapeutic treatment (except for steroids) was allowed within 2 weeks of administration of As2O3.
Evaluation of toxicity and disease progression
Disease progression and response was evaluated using the new international criteria proposed by the Response Evaluation Criteria in solid Tumors (RECIST) committee Citation[6]. EKG was obtained biweekly during the first week of treatment, then weekly, and as clinically indicated. History and physical examination were performed weekly. Hematology and chemistry profiles were done biweekly. Pre and post treatment carcinoembryonic antigen (CEA) levels were also monitored. CT scan of the affected area was performed at the beginning and at the end of each cycle of the therapy. End of therapy evaluation included history and physical exam, hematology and chemistry profiles, CEA measurement, EKG and urinalysis, imaging and diagnostic studies (if indicated) and tumor response assessments. To be assigned a status of PR or CR, changes in tumor measurements must be confirmed by repeat assessments that should be performed 4 weeks after the criteria for response are first met. In the case of SD, follow-up measurements must have met the SD criteria at least once after study entry at a minimum interval of 6 weeks.
Correlative laboratory studies to analyze the effect of arsenic trioxide on the expression of TS mRNA
Peripheral blood samples were collected from the study participants prior to and 24 hours post arsenic administration. White blood cells were isolated from the peripheral blood as described previously Citation[7]. Total RNA from WBC was isolated using Tri-Reagent (Sigma, USA). The sequences of primers used to quantitate TS was TS 5’ Primer A–(24 mer) 5′-GGGCAGATCCAACACATCCTCCGC-3′ and TS Rev. Primer (20 mer)-5′-GCCCAAGTCCCCTTCTTCTC-3′ that synthesized a 294 bp long product. β-Actin F-(25 mer) 5′-TCACCCACACTGTGCCCATCTACGA-3′ and β-Actin R-(25 mer)-5′-CAGCGGAACCGCTCATTGCCAATGG-3′ synthesized a 295 bp fragment which also served as the internal control. These primers spanned the exon-intron junction so that genomic DNA was not amplified. The list of specialty chemicals used, details of cDNA synthesis by reverse transcription and RT-PCR procedure are as described in Sarkar et al. Citation[8]. Each reaction was performed at two different dilutions of the template. The mean normalized expression (MNE) was calculated as detailed in Muller et al. Citation[9].
Results and discussion
There are no treatment options available for patients with refractory metastatic colorectal cancer. Of late, arsenic trioxide had been shown to cure APL [1;3]. It also showed promising results in multiple myeloma patients Citation[5]. Preclinical studies demonstrated the efficacy of arsenic trioxide on various cultured colorectal cancer cell lines. This led us to initiate this Phase II clinical trial to assess the anti tumor activity and toxicity of arsenic trioxide in patients with refractory metastatic colorectal carcinoma.
Five patients (three males and two females) were enrolled in this clinical trial between May and October of 2002 at the Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, USA. The median age was 60 that ranged from 41 to 78 years. All of them were evaluable for response and toxicity. Every one had evidence of progressive disease, as documented by CT scans prior to therapy. They had undergone prior surgeries and chemotherapies. Under this trial three patients completed at least one cycle, while two others received more than two cycles of chemotherapy.
All the patients developed moderate to severe toxic side effects. A list of toxicities encountered is detailed in . The most common side effects reported were fatigue, nausea/vomiting, dehydration, neuropathy, thrombocytopenia and anemia. One of the patients developed Herpes zoster and another had a relapse of Herpes genitalis that resolved with antiviral treatment. Occurrence of herpes infection in patients under arsenic therapy had been reported. It appears that arsenic weakens immune system Citation[10]. Due to personal reasons one patient opted out who was latter diagnosed with CNS metastasis. In four patients the CEA level remained stable while, in one it increased by 3.4 fold (). Across the group, a moderate decrease in hemoglobin level, WBC and RBC counts were observed. In one patient, the platelet count doubled. One of the patients showed mild changes in QTc that eventually returned to normal. CT scan was performed at the end of the treatment cycle to evaluate disease. Due to lack of clinical response as monitored by CT scan and severe toxicity in all the five patients, this study was aborted after two cycle of treatment. Thus, arsenic trioxide/ascorbic acid therapy did not produce desirable outcome in this group of heavily pretreated patients.
Table I. Toxicity
Table II. Data from some of the analyzed hematological parameters
Two of the participants were alive at the time of last follow-up. The remaining three died within 2 to 5 months after withdrawing from the study. None of the deaths could be attributed to arsenic trioxide administration.
Toxic side effects of arsenic trioxide were a major concern. A previous phase I study demonstrated 0.25 to 0.35 mg/kg/day to be acceptable dose Citation[11]. In this study, each patient received five doses of arsenic trioxide/ascorbic acid a week for 5 consecutive weeks. We believe that down sizing the treatment regimen might lessen the toxicity.
The lack of clinical response and pronounced side effects in this trial is intriguing but not without precedence. A similar study involving renal cell carcinoma (RCC) Citation[12], metastatic melanoma Citation[13] and refractory germ cell malignancies Citation[14] also did not produce any clinical response. It appears that solid tumors do not respond to arsenic, which affects oxidative phosphorylation pathway thereby killing the cells Citation[2]. Most of the solid tumors are believed to be hypoxic Citation[15]. Therefore, even if delivered, due to their anaerobic lifestyle hypoxic tumors might be least affected by arsenic. Besides, by virtue of the route of administration, hematopoietic cancer cells might be in direct contact with As2O3. This direct physical contact may augment the effectiveness of treatment. In contrast inner core of tumors lack blood vessels and therefore may not receive As2O3 /AA. Thus, non-delivery of arsenic trioxide to solid tumors may be a reason for the treatment failure. Therefore, the problem of delivery of drugs to inner core of the tumor needs to be addressed.
Arsenic affected TS transcription
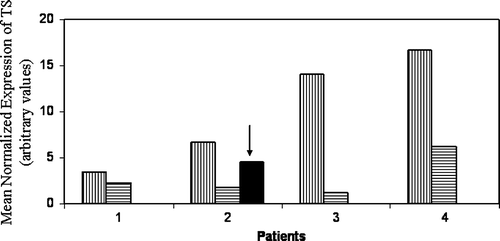
The standard colorectal cancer chemotherapy regimen includes 5-FU in combination with other drugs like CPT-11, Leucovorin etc. However, a majority of patients eventually fail to respond to this line of treatment. This limits the treatment options available for refractory cases. Our aim is to find agents that could sensitize refractory CRC to 5-FU. It interferes with thymidylate synthase (TS), a key enzyme for the conversion of deoxyuridylate to deoxythymidylate. In general the TS expression RNA is found elevated in 5-FU refractory tumor samples. Lowering of the TS quantity in resistant cells restored their sensitivity to 5-FU Citation[16] (Subbarayan et al. unpublished observations). Therefore, we quantitated TS message in the peripheral blood leukocytes in the patients following arsenic trioxide/ascorbic acid administration. The result of this study is presented in . We found that following treatment TS mRNA expression was significantly reduced (p = 0.03, one-sided paired t-test) in the peripheral blood mononuclear cells (PBMC) of the four patients from whom blood samples were obtained. There is inter-patient variation in the expression of TS and in response to treatment. TS transcripts in PBMC decreased from 37% to 92% after treatment with arsenic (with a median value of 63%). In one patient who was available for post treatment evaluation, within seven days after the cessation of arsenic treatment, the TS message level was restored to 67% of the pretreatment level (, Arrow). When TS level is reduced the tumor might have been sensitized back to 5-FU. Therefore, a combination of sub-toxic dose of As2O3 with 5-FU may restore sensitivity in refractory tumors. This strategy can form a basis for As2O3/5-FU combination chemotherapy.
Figure 1. TS transcript level decreased with arsenic treatment. Peripheral blood samples were drawn prior to (vertical bars) and post arsenic infusion (horizontal bars) and Mean Normalized Expression of TS mRNA was measured as described in materials and methods. Real time PCR analysis of TS and β-actin expression was done as described under materials and methods. Recovery of TS during two weeks of intermission in treatment is highlighted with an arrow.
In conclusion, since there were severe toxic side effects to 0.25 mg/kg/day dose, we suggest that a judicious combination of low or dose of As2O3 and a modified treatment regimen could lower TS level in tumor cells with less severe toxic side effects. It may also open a way for the use of other chemotherapy agents in combination with arsenic trioxide.
Conflict of interest statement
None declared
Acknowledgements
Dedicated to the memory of late Dr. Benito Que.
References
- Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 1997; 89: 3345–53
- Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res 2002; 62: 3893–903
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 2001; 19: 3852–60
- Chu E, Allegra CJ. Mechanisms of clinical resistance to 5-fluorouracil chemotherapy. Cancer Treat Res 1996; 87: 175–95
- Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, Kharfan-Dabaja M, et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res 2002; 8: 3658–68
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16
- Subbarayan PR, Sarkar M, Ardalan B. Isolation of genomic DNA from human whole blood. Biotechniques 2002; 33: 1231–1234
- Sarkar M, Han T, Damaraju V, Carpenter P, Cass CE, Agarwal RP. Cytosine arabinoside affects multiple cellular factors and induces drug resistance in human lymphoid cells. Biochem Pharmacol 2005; 70: 426–32
- Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 2002; 32: 1372–9
- Nouri K, Ricotti CA, Jr, Bouzari N, Chen H, Ahn E, Bach A. The incidence of recurrent herpes simplex and herpes zoster infection during treatment with arsenic trioxide. J Drugs Dermatol 2006; 5: 182–5
- Soignet SL, Bienvenu B, Cheund N-K, et al. Clinical and pharmacologic study of arsenic trioxide (As2O3) in patients with solid tumors (abstract). Proc Am Soc Clin Oncol 2000; 19: 201a
- Vuky J, Yu R, Schwartz L, Motzer RJ. Phase II trial of arsenic trioxide in patients with metastatic renal cell carcinoma. Invest New Drugs 2002; 20: 327–30
- Kim KB, Bedikian AY, Camacho LH, Papadopoulos NE, McCullough C. A phase II trial of arsenic trioxide in patients with metastatic melanoma. Cancer 2005; 104: 1687–92
- Beer TM, Tangen CM, Nichols CR, Margolin KA, Dreicer R, Stephenson WT, et al. Southwest Oncology Group phase II study of arsenic trioxide in patients with refractory germ cell malignancies. Cancer 2006; 106: 2624–9
- Boyle RG, Travers S. Hypoxia: Targeting the tumour. Anticancer Agents Med Chem 2006; 6: 281–6
- Ju J, Kane SE, Lenz HJ, Danenberg KD, Chu E, Danenberg PV. Desensitization and sensitization of cells to fluoropyrimidines with different antisenses directed against thymidylate synthase messenger RNA. Clin Cancer Res 1998; 4: 2229–36
