Abstract
Humoral immune responses to the MUC1 peptide and to MUC1-related Thomsen-Friedenreich (TF) glycotope was investigated in patients with gastric cancer (n = 247), chronic gastroduodenal diseases (n = 199) and in healthy blood donors (n = 100). Data were correlated with disease type, stage of cancer, tumor morphology and survival. MUC1 IgG antibody levels were higher in patients with gastric cancer (p < 0.0001) than in healthy controls. Higher levels of anti-MUC1 IgG were also detected in patients with ulcer of the stomach (p = 0.015) and in atrophic gastritis (p = 0.027). Compared to blood donors, significantly lower levels of anti-TF IgG were found both in the cancer (p = 0.002) and in the benign group (p < 0.0001). At early stages of cancer a positive correlation (p < 0.0001) was found between MUC1 IgG and TF IgG antibody levels. High levels of TF IgG antibodies were significantly associated with a benefit in survival of gastric cancer patients (p = 0.003). A similar though weaker association was observed for patients with high levels of MUC1 IgG antibodies and locoregional disease (stage I-III) (p = 0.037). Thus IgG immune responses to MUC1 are increased in patients with gastric cancer. High levels of either TF IgG or MUC1 IgG antibodies may predict better outcome in surgically treated patients with gastric cancer.
MUC1 is a high-molecular weight (over 400 kD) membrane-bound glycoprotein with a highly glycosylated extracellular domain that consists of tandemly repeated highly conserved sequences of 20 amino-acids (TSAPDTRPAPGSTAPPAHGV) that is expressed on the ductal cell surface of normal glandular epithelia Citation[1], Citation[2]. Overexpression of MUC1 in the majority of adenocarcinomas Citation[3] diminishes the adhesive properties of tumor cells, thus favouring tumor progression and metastases Citation[4–6]. In gastric carcinomas, MUC1 expression is associated with poorer outcome Citation[5], Citation[6]. Anti-MUC1 antibodies (MUC1 Ab) directed to the peptide core of the molecule are present in patients with various cancers and in healthy individuals Citation[8], Citation[9]. A high level of anti-MUC1 antibodies or circulating immune complexes containing MUC1 was associated with a significantly better disease free survival in early breast cancer patients Citation[10] and in non-small cell lung cancer Citation[12].
Several tumor-associated mucin-type O-glycosyl-linked carbohydrate epitopes, such as Thomsen-Friedenreich (TF) antigen (Galβ1-3GalNAcα/β-O-Ser/Thr) and Tn antigen (GalNAcα1-O-Ser/Thr), are expressed on tumor-associated MUC1 due to incomplete glycosylation of the molecule Citation[7], Citation[13]. Immune responses to underglycosylated MUC1 are polyclonal and may be directed not only to the molecule's core peptide but also to the tumor-associated glycans expressed on it Citation[11]. Natural antibodies to these glycotopes are present in every individual, and their level is decreased in cancer patients Citation[14], Citation[15]. It has been shown that some anti-carbohydrate antibodies (anti-alpha-Gal, anti-Le Y) may interact with peptide epitopes including MUC1 peptide Citation[16], Citation[17]. On the other hand, some peptides show high affinity to carbohydrate epitopes, for instance to TF antigen Citation[18]. These observations suggest that carbohydrate epitopes are able to mimic structurally and functionally peptide epitopes. The relationship between immune responses to the MUC1 peptide core antigen and to MUC1-related carbohydrate epitopes remains unclear.
The objective of this study was to concurrently investigate the level of anti-MUC1-peptide antibodies and the immune response to tumor-associated carbohydrate TF antigen, which is expressed on the MUC1 molecule. Another tumor-related alpha-Gal glycotope (Galα1-3Galβ1-4GlcNAc-R) which is expressed mostly on glycolipids or N-linked carbohydrate chains Citation[19] was used as non-mucin type glycotope for comparison. In addition, we assessed a possible association between the level of anti-MUC1, anti-carbohydrate antibodies and clinical characteristics, including the survival of patients with gastric cancer.
Material and methods
Subjects and serum samples
Serum samples were obtained from patients with histologically verified gastric carcinoma (n = 247 and patients with non-malignant gastroduodenal pathology (n = 199). In addition we tested serum samples obtained from a group of randomly selected blood transfusion donors (n = 100). All patients and controls were over 40 years old. Tumor staging and morphology were based on histopathological (pTNM) classification of malignant tumors and evaluated according to the system of Lauren, 1965 Citation[20] as intestinal (n = 155) and diffuse type (n = 87) of tumor growth (five patients with mixed tumors, which were difficult to classify, were not included in the analysis). Peptic ulcer disease was diagnosed by gastroduodenal endoscopy. Antrum and corpus biopsy specimens were assessed histologically, and moderate or severe gastric mucosa atrophy in any part of the stomach was considered as atrophic gastritis.The distribution of cancer patients by stage and other characteristics is presented in .
Table I. Antibody levels to MUC1, TF and αGal glycotope in patients and controls.
Serum samples were obtained before treatment and stored at −20°C until required.
Detection of IgG and IgM immune response to MUC1
IgG and IgM antibody levels to MUC1 (MUC1 Ab) were determined with ELISA as described elsewhere Citation[21]. In brief: a BSA-conjugated MUC1 60-mer tandem-repeat peptide (250 ng per well in PBS) and 1% BSA (control) were used to coat 96-well ELISA plates (Maxisorp, Nunc, Roskilde, Denmark). After overnight incubation at 4°C, washing and blocking with 1% BSA in PBS the serum diluted 1:100 and 1:500 for IgG and IgM antibody determination, respectively, was applied and the plates were incubated overnight at 4°C. After 7× washing with PBS-Tween 20 the bound antibodies were detected with alkaline phosphatase conjugated rabbit anti-human IgG or IgM (Dako) and developed with p-nitro-phenyl-phosphate (Sigma). Absorbance values were registred at 405 nm with Labsystem Multiscan (Finland). An optical density of control wells (PBS-BSA) was subtracted from the values of the wells coated with MUC1-BSA.To standardize the assay, a serum sample with a level of anti-MUC1 IgG of about 1.0 O.D. unit was included as a standard in every plate. The tested serum value was calculated as a percentage of the value of the standard serum (100%) and expressed in relative units (R.U.).
Anti-TF and anti-αGal antibody assay (TF-Ab and αGal-Ab)
Plates (Maxisorp, Nunc) were coated with synthetic TF- or αGal-hapten-polyacrylamide (PAA) conjugate (Lectinity, Russia, 10 mol% of carbohydrates for TF-PAA and 20 mol% for αGal-PAA conjugate) 5 µg/ml in carbonate buffer, pH 9.6 or 1% BSA in PBS (control wells) at 4°C overnight. After washing three times with PBS-0.05% Tween-20, 0.05 mM EDTA, the plates were blocked with 1% BSA in PBS for 1 h at room temperature (RT) and washed with PBS-Tween. Serum (100 µl) diluted 1:50 in PBS-Tween-EDTA was added and incubated for 2 h at 26°C. After 3× washing, bound IgG was detected with alkaline phosphatase-conjugated rabbit anti-human IgG (Dako, Denmark) followed by p-nitro-phenyl-phosphate (Sigma, St.Louis, MO). For TF IgM determination, serum dilution 1:500 and alkaline phosphatase-conjugated rabbit anti-human IgM (Dako, Denmark) were used. Optical density (O.D.) values of control wells were subtracted from the values of wells coated with hapten-PAA conjugates. Two reference sera from weak and strong responders were run in every plate as an internal standard. Each serum was analysed in duplicate. The intra- and inter-assay variations did not exceed 9.8%.
Statistical methods
Results were analysed for normality of distribution and comparisons between the groups were performed with the Mann-Whitney U-test and Pearson two-tailed correlation. The median of TF, αGal or MUC1 antibody levels (O.D. or R.U. values) measured in the gastric cancer group was used as cut-off to define strong and weak responders: Patients with antibody levels equal or greater than the corresponding median O.D. or R.U. value were classified as strong responders, and those with levels below the median as weak responders. The survival of cancer patients with weak and strong response to MUC1 and glycotopes was analysed by the Kaplan-Meier method (log-rank test). A difference between groups was considered to be significant when p ≤ 0.05. All calculation were performed using GraphPad Prisma 4 software.
Results
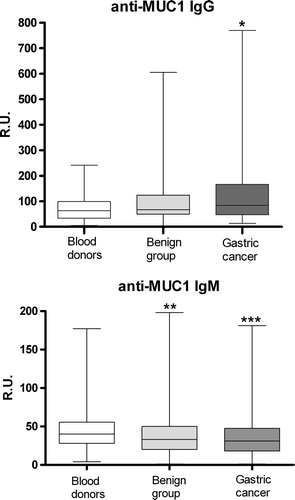
Anti-MUC1 antibodies of both IgG and IgM isotypes were present in serum of patients and controls. When compared to blood donors, a significantly higher level of IgG antibody to MUC1 was found in patients with gastric cancer (p = 0.0002) (). With one exception, no differences in MUC1 IgG Ab levels in relation to tumour morphology (diffuse vs. intestinal type) was found: MUC1 IgG levels did rank significantly higher in patients with advanced disease (stage III and IV) and diffuse tumours (p = 0.0007). A high level of IgG antibody to MUC1 was noted already in patients with stage I disease (p = 0.0002), with no significant relation to the further progression of the disease.
Figure 1. MUC1 IgG and IgM antibody levels (medians, ranges and quartiles) in patients with gastric cancer (n = 247), chronic gastroduodenal diseases (n = 199) and blood donors (n = 100) p-values are calculated by Mann-Whitney U test. *significantly higher than in blood donors (p < 0.0001); **significantly lower compared to blood donors group (p = 0.005); ***significantly lower compared to blood donors group (p = 0.0002).
Compared to blood donors, an increase of MUC1 IgG Ab, albeit less pronounced than in patients with gastric cancer, was found in patients with stomach ulcer (p = 0.015) and with chronic atrophic gastritis (p = 0.027). No significant difference in MUC1 IgG Ab levels was found between patients with duodenal ulcer or non-atrophic gastritis and controls. A significant difference in ranking of MUC1 IgG Ab levels between cancer patients and benign gastric diseases was found only for patients with duodenal ulcer (p = 0.04), being higher in cancer patients.
Unlike MUC1 IgG antibodies, the levels of IgM antibodies to MUC1 were significantly lower in patients with cancer compared to donors (p = 0.0002) (). Among patients with chronic gastric diseases only patients with duodenal ulcer showed a highly significant decrease of MUC1 IgM level (p = 0.0004). No significant correlation was found between the levels of IgG and IgM antibody for any of the groups studied.
In contrast to MUC1 IgG antibody levels, the levels of antibodies to TF glycotope in patients with gastric cancer were lower than in controls irrespective of stage of disease (). However, even lower levels of TF IgG antibodies than in controls was observed in all groups of patients with benign gastric diseases (p < 0.0001). This was also true for TF IgM antibody levels. Moreover, the level of TF IgM antibodies in all subgroups of patients with benign gastroduodenal diseases was significantly lower that in patients with various stages of gastric cancer (p < 0.001–0.002).
Compared to the control group, IgG immune responses to αGal epitope were significantly higher in patients with benign gastric diseases (p = 0.03 for combined benign group) reaching statistical significance only in the subgroup of patients with duodenal ulcer (p = 0.05). Neither TF nor αGal antibody levels were related to the stage or morphology of gastric tumors.
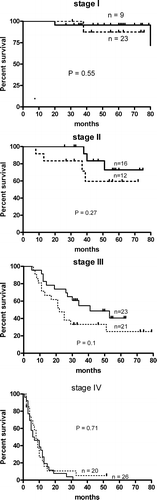
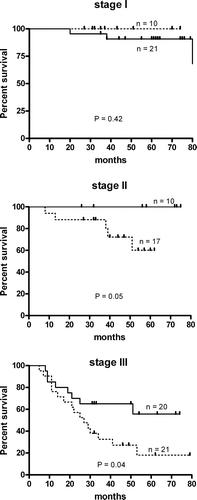
A positive correlation was found between anti-MUC1 IgG and anti-TF IgG levels in patients with stage I disease (r = 0.4; n = 64, p < 0.01) as well as for the combined group of gastric cancer patients (stage I + Stage II; r = 0.47, p = 0.0001) (). No such association was found in any of the other groups studied including patients with advanced gastric cancer. Patients with TF IgG antibody above the median value (strong responders) had a highly significant benefit in survival compared to weak TF responders for the combined groups (all stages and patients with locoregional stage I-III of the disease (p = 0.003 and p = 0.005 respectively) (A,B).
Figure 2. Correlation between the levels of MUC1 IgG and TF IgG antibodies for stage I + stage II patients with gastric cancer.
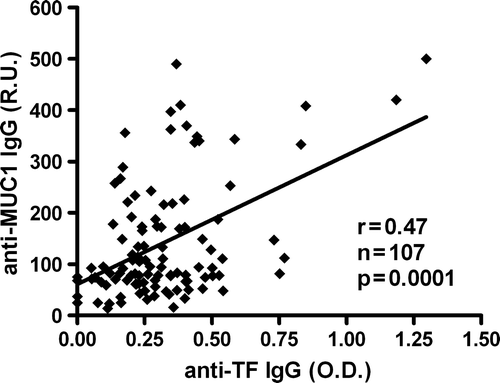
Figure 3. Probability of survival (Kaplan-Meier method) of gastric cancer patients in relation to TF IgG (A, B) and MUC1 IgG (C, D) antibody levels: (combined groups: stage I-IV (A, C) and stage I-III (B, D) Dark line – strong responders (O.D. or R.U. values above the median); Dotted line – weak responders (O.D. or R.U. values below or equal to the median).
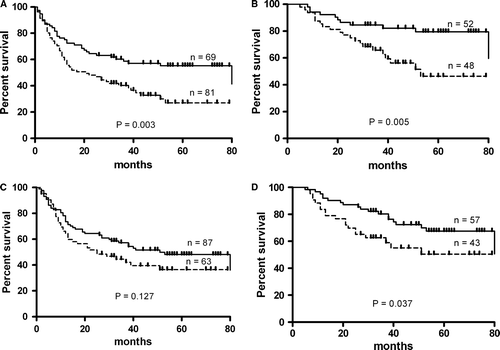
A drastic difference in the median survival time of stage I-IV patients was observed: 83 and 21 months for strong and weak responders respectively; hazard ratio 1.87, 95% CI of ratio 1.25–2.94. The difference for stage I-III patients was 83 and 53 months respectively; hazard ratio 2.68; 95% CI 1.37–5.78, and p = 0.005. Similar analysis for MUC1 IgG antibody (C,D) showed a significant association with survival only for stage I-III patients (p = 0.037). In both cases these associations were mostly related to patients with stage II and stage III, and were more pronounced for TF IgG antibody ( and ). No differences in survival was demonstrated in relation to MUC1 IgM or TF IgM antibodies (data not shown). The level of αGal specific IgG antibodies was not related to the survival as well.
Discussion
In agreement with other reports we found that serum antibodies to MUC1 are present in both cancer patients and controls Citation[8], Citation[9]. However, patients with gastric cancer revealed significantly higher level of MUC1 IgG antibodies compared to healthy blood donors. This increase was in part dependent on the histological type of tumor growth being more pronounced in patients with advanced diffuse type tumors. This might be due to the higher expression of MUC1 in low differentiated tumors Citation[7] or to a degree of its deglycosylation. However, in some malignancies (non-small cell lung cancer), the level of MUC1 antibodies was found to be significantly lower than in normal individuals Citation[12]. The increase of MUC1 IgG antibody level in patients with gastric cancer did not show strong stage-dependency, suggesting that an acquired IgG immune response to MUC1 appears at early stages of cancer and does not change appreciably during the progression of the disease. The absence of correlation between MUC1 IgG and MUC1 IgM antibody levels indicates that the up-regulation of the IgG immune response does not coincide with the changes in IgM antibody levels in a majority of patients. Possibly, the IgM and IgG levels reflect different, partially independent arms of the response i.e. innate and adaptive.
Cancer patients with higher level of MUC1 IgG antibody showed better survival suggesting that anti-MUC1 IgG immune responses may be beneficial for the patient when the primary tumor is removed. This is in accordance with the findings in patients with breast and lung cancer where higher MUC1 Ab levels were associated with a better survival Citation[10], Citation[12]. An increase in MUC1 IgG antibody level in patients with gastric cancer was about two times less than in patients with breast cancer (Kurtenkov et al., unpublished data). This indicates that the expression of MUC1 and an induction of immune response to this epitope may differ in various cancers. Nevertheless, this weak association with survival in gastric cancer patients is an additional indication that vaccination in an adjuvant setting with MUC1 substrates (preferably glycopeptides carrying the TF antigen) to induce a strong immune response may be beneficial and prolong survival.
In contrast to MUC1 IgG antibody, anti-TF IgG and IgM antibody levels were significantly decreased in patients with non-malignant diseases and cancer with no relation to the stage of cancer. It has been shown that individuals with low level of TF Ab are at higher risk for cancer development Citation[14]. This, as well as the absence of stage-dependency, is an argument for the idea that low level of TF Ab in cancer patients may be due to the overrepresentation of low (genetically conditioned) TF responders among patients with cancer, and that lower levels of anti-TF antibodies preceed malignancy. This does not exclude, however, that strong responders among patients with cancer may be actually those who have an enhanced acquired immune response to tumor derived TF. We have shown that the level of TF Abs in healthy individuals is fairly stable over time Citation[22] and unpublished data] suggesting that it is rather conserved, as it has been shown for natural self-reactive antibody levels Citation[23]. In any case, the association of higher level of TF IgG antibody with a better survival suggests that naturally occurring or adaptive immune responses to this epitope are of clinical importance and may be beneficial for the host. Furthermore, induction of immune responses to TF by vaccination seems a plausible adjuvant treatment for gastric cancer.
A significant positive correlation was found between the levels of TF IgG and MUC1 IgG antibodies in patients with early stages of gastric cancer but not in other groups tested. A moderate correlation implies that it is related to a small (sub)population of the patients; as it can be seen in . The association of these immune responses with early stage disease suggests that they could play a role in limiting tumor spread, as observed in breast cancer Citation[10]. Exposure of the MUC1 peptide core is due to underglycosylation of the molecule, which also leads to the expression of TF glycotope, linked to a motif within the MUC1 repeat Citation[7]. Therefore, altered (incomplete) glycosylation, which is a well known general phenomenon associated to tumor proteins, leads to the expression of cryptic epitopes involved in these immune responses. At the same time no association was found between immune response to MUC1 and MUC1 non-related αGal epitope.
The finding that association with a better survival of gastric cancer patients with high levels of TF IgG was more pronounced than that of patients with high MUC1 antibody levels suggests that the effect on survival may be rather related to the underglycosylation of MUC1 mucin molecule in cancer patients and the induction of immune response to the simple-type MUC1 related glycotopes, such as TF, than to MUC1 core epitope itself. In patients with gastric cancer, an association with the latter was rather weak and awaits further confirmation. Thus, it is unclear whether the MUC1 expression per se is critical for this effect, or the association with survival is related to the overexpression of TF epitope on MUC1 or other mucins and glycoproteins frequently overexpressed and altered in patients with cancer.
The polymorphism in the MUC1 tandem repeat influences the expression of TF antigen in gastric cancer cells Citation[24]. This might allow the identification of subgroups of patients that develop more aggressive tumors expressing TF antigen. There is evidence that the specificity of tumor-derived MUC1 as well as its antigenicity are related to the glycosylation pattern of MUC1 Citation[25]. The differences in MUC1 glycosylation was recently shown to be important for the host humoral immune response polymorphism Citation[26] suggesting that immune response to MUC1 may contribute to the diversity of tumor-host immunological relationships and the clinical outcome of the disease.
In conclusion, IgG immune response to MUC1 is significantly enhanced in patients with gastric cancer. High level of both TF IgG and MUC1 IgG antibody may predict better outcome in surgically treated patients with gastric cancer. The data further support the idea that the MUC1 and mucin type tumor-related glycotopes can be considered as potential tumor targets for cancer immunotherapy.
We thank Mrs. Tatjana Djomina for technical assistance.This study was supported by a grant #5436 and #6726 from the Estonian Science Foundation.
References
- Price MR, Rye PD, Hudecz F, O'Sullivan C, Baldwin RW, Edwards PM, et al. Immunological and structural features of the protein core of human polymorphic epithelial mucin. Molec Immunol 1990; 27: 795–802
- Von Mensdorff-Pouilly S, Snijdewint FGM, Verstraeten AA, Verheijen RHM, Kenemans P. Human MUC1 mucin: A multifaceted glycoprotein. J Biol Markers 2000a; 15: 343–56
- Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochim Biophys Acta 1999; 1455: 301–13
- Hilkens J, Wesseling J, Vos HL, Storm J, Boer B, van der Valk SW, et al. Involvement of the cell-surface bound mucin, episialin/MUC1, in progression of human carcinomas. Biochem Soc Transact 1995; 23: 822–6
- Denda-Nagai K, Irimura T. MUC1 in carcinoma host interactions. Glycoconj J 2000; 17: 649–58
- Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH. MUC1, MUC2, Muc5AC, and MUC6 expressions in gastric carcinomas: Their role as prognostic indicators. Cancer 2001; 92: 1427–34
- Baldus SE, Hanisch FG, Monaca E, Karsten UR, Zirbes TK, Thiele J, et al. Immunoreactivity of Thomsen-Friedenreich (TF) antigen in human neoplasms: The importance of carrier-specific glycotope expression on MUC1. Histol Histopathl 1999; 14: 1153–6
- Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immune immunity against a tandem repeat of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res 1994; 54: 2856–60
- Richards ER, Devine PL, Quin RJ, Fontenot JD, Ward BG, McGuckin MA. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol Immunother 1998; 46: 245–52
- Von Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FGM, Kok A, van Kamp GJ, et al. Survival in early breast cancer patients is favourably influenced by a natural humoral immune response to polymorphic epitheliual mucin (MUC1). J Clin Oncol 2000b; 18: 574–83
- von Mensdorff-Pouilly S, Petrakou E, Kenemans P, Van Uffelen K, Verstraeten AA, Snijdewint FGM, et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and N-acetylgalactosamine (GalNAc) peptides. Int J Cancer 2000; 86: 702–12
- Hirasawa Y, Kohno N, Yokoyama A, Kondo K, Hiwada K, Miyake M. Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am J Resp Crit Care Med 2000; 161: 589–94
- Schuman J, Campbell AP, Koganty RR, Longenecker BM. Probing the conformational and dynamical effects of O-glycosylation within the immunodominant region of a MUC1 peptide tumor antigen. J Pept Res 2003; 61: 91–108
- Springer GF. T and Tn, general carcinoma autoantigens. Science 1984; 224: 1198–206
- Kurtenkov O, Miljukhina L, Smorodin J, Klaamas K, Bovin N, Ellamaa M, Chuzmarov V. Natural IgM and IgG antibodies to Thomsen-Friedenreich(T) antigen in serum of patients with gastric cancer and blood donors. Acta Oncol 1999; 38: 939–43
- Apostolopoulos V, Sandrin MS, McKenzie IF. Mimics and cross reactions of relevance to tumour immunotherapy. Vaccine 1999; 18: 268–75
- Luo P, Canziani G, Cunto-Amesty G, Kieber-Emmons T. A molecular basis for functional peptide mimicry of a carbohydrate antigen. J Biol Chem 2000; 275: 16146–54
- Petelskaya EN, Glinsky VV, Glinsky GV, Deutscher SL, Quinn TP. Characterization of peptides that bind the tumor-associated Thomsen-Friedenreich antigen selected from bacteriophage display libraries. J Mol Biol 1997; 270: 374–84
- Galili U, LaTemple DC, Radic MZ. The natural anti-Gal antibody. Subcellular Biochemistry 1999; 32: 79–106
- Lauren P. The two histological main types of gastric carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49
- Von Mensdorff-Pouilly S, Gourevitch MM, Kenemans P, Verstraeten AA, van Kamp GJ, Uffelen K, et al. An enzyme-linked immunosorbent assay for the measurement of circulating antibodies to polymorphic epithelial mucin (MUC1). Tumor Biol 1998; 19: 186–95
- Kurtenkov O, Klaamas K, Miljukhina L. The lower level of natural anti-Thomsen-Friedenreich antigen (TFA) agglutinins in sera of patients with gastric cancer related to ABO(H) blood.group phenotype. Int J Cancer 1995; 60: 781–5
- Lacroix-Desmazes S, Mouthon L, Kaveri SV, Kazatchkine MD, Weksler ME. Stability of natural self-reactive antibody repertoires during aging. J Clin Immunol 1999; 19: 26–34
- Santos-Silva F, Fonseca A, Caffrey T, Carvalho F, Mesquita P, Reis C, et al. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology 2005; 15: 511–7
- Karsten U, Serttas N, Paulsen H, Danielczyk A, Goletz S. Binding patterns of DSTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1). Glycobiology 2004; 14: 681–92
- von Mensdorf-Pouilly S, Kinarsky L, Engelmann K, Baldus SE, Verheijen RH, Hollingsworth MA, et al. Sequence variant repeats of MUC1 show higher conformational flexibility, are less densely O-glycosylated and induce differencial B lymphocyte response. Glycobiology 2005; 15: 735–46