Abstract
To report the long-term results for treatment of localized carcinoma of the prostate using high dose rate (HDR) brachytherapy, conformal external beam radiotherapy (3D EBRT) and neo-adjuvant hormonal therapy (TAB). From 1998 through 1999, 154 patients with localized prostate cancer were entered in the trial. Biologically no evidence of disease (bNED) was defined at PSA levels < 2 µg/l. In order to compare the results of this treatment with other treatment modalities, the patient's pre-treatment data were used to calculate the estimated 5-year PSA relapse free survival using Kattan's nomograms for radical prostatectomy (RP) and 3D EBRT. After 6 years of follow-up, 129 patients remain alive. The actual 5-year relapse-free survival is 84%. None of the patients demonstrated clinical signs of local recurrence. The median PSA at follow-up among the relapse-free patients was 0.05 µg/l. Among the 80 patients who presented with clinical stage T3 tumours, 55 (68%) were relapse-free. The expected 5-year relapse-free survival using nomograms for RP and 3D EBRT was 54% and 70%, respectively. Late rectal toxicity RTOG grade 3 occurred in 1% of the patients. Late urinary tract toxicity RTOG grade 3 developed in 4% of the patients. Combined treatment, utilizing HDR, 3D EBRT and TAB, produces good clinical results. Rectal toxicity is acceptable. Urinary tract toxicity, most likely can be explained by the fact that during the first years of this treatment, no effort was made to localize the urethra, which was assumed to be in the middle of the prostate.
In Sweden, prostatic adenocarcinoma is the most common male cancer, with an annual incidence of 168/100 000 Citation[1]. The optimal method of treatment for patients with localized prostate cancer remains to be defined, since there are several approaches to treatment such as radical prostatectomy, radiotherapy with curative intention or watchful waiting. Furthermore the optimal method of radiation therapy, external beam radiotherapy, brachytherapy (low dose rate or high dose rate), or a combination, remains in question. The currently accepted standard is that the curative dose of irradiation should be 70 Gy or greater, and several dose-escalating projects are currently investigating the optimal dose for cure and minimal side effects.
Known side effects when using external beam radiotherapy include symptoms from the rectum and/or the urinary bladder caused by the dose of irradiation to these organs which are in close proximity to the prostate.
The advantage of brachytherapy is the short range of its irradiation, leading to reduced doses of irradiation to the rectum and bladder. However, this short range may also produce the problem of inhomogeneous dose-distributions and the risk of insufficient dose to parts of the target. Another problem is that the source must be accurately placed in the target or in very close proximity.
Since 1998, we have utilized a treatment method, which combines neo-adjuvant hormonal therapy, conformal external beam radiotherapy (EBRT), and two sessions of high dose rate (HDR) iridium 192 source brachytherapy for patients with localized prostate cancer. The principle of this treatment has been reported previously Citation[2]. This combination delivers a normalized dose equivalent of 2 Gy per fraction (NTD) of more than 104 Gy (assuming a conservative tumour alpha/beta ratio = 3 Gy). Presently, the patient-reported toxicity and quality of life up to 3 years after treatment seem acceptable Citation[3], Citation[4].
In this article, clinical outcome and side effects at a median follow-up of 6 years will be reported and compared with the calculated results using accepted nomograms for radical prostatectomy and dose escalated conformal external beam radiotherapy.
Material and methods
Patients
From May 1998 through December 1999, 154 patients with localized prostate cancer entered this trial. The patients had a biopsy proven prostatic adenocarcinoma. The Karolinska has a long tradition of fine needle aspiration cytology (FNAC). Ultrasound guided core needle biopsies in the diagnosis of prostate cancer was introduced relatively late at the hospital. Briefly, with FNAC dispersions of isolated or groups of cells are assessed according to the morphology of nuclei and cytoplasm whereas core biopsies provide tissue pieces suitable for histopathological examination. Consequently, in the initial period of the protocol most patients were diagnosed only with FNAC. The biopsies were reported according to the WHO grading system and later the Gleason system. The Gleason score was transformed to WHO high, middle or poor differentiation defining Gleason score ≤5 as denoting low grade cancer and Gleason score ≥7 (4 + 3) denoting high grade cancer. The T-stage was defined according to the 1997 TNM classification system.
A lymph node dissection was performed if the tumour was high grade or the prostate-specific antigen in serum (PSA) exceeded 20 µg/l. Only patients with negative lymph node sampling were included. In this report, 103 patients were surgically staged with negative biopsies of lymph nodes. A bone-scan was routinely performed if the PSA exceeded 10 µg/l, in order to exclude bone metastasis. All patients received neo-adjuvant hormonal (TAB) treatment for 6–9 months, consisting of anti testosterone (orally Bicalutamide 50 mgx1 or Flutamide 250 mg×3) combined with subcutaneous implants of a gonadotropin releasing hormone analogue.
Patient characteristics at diagnosis are listed in . Pre-treatment PSA was missing in one patient and T-stage was not assessed in one patient.
Table I. Patient characteristics covering age at diagnosis, PSA levels, differentiation grade according to WHO, T-stage and N-stage.
Poor prognostic risk factors were defined as follows: PSA >10 µg/l, T-stage 3 (TNM), and poorly differentiated prostate cancer (WHO grade III). 32 patients had no risk factors, 54 patients had one risk factor, 52 patients had two risk factors and 14 patients had all three risk factors.
All patients have been identified for follow-up and 24 have died, however, four patients have moved abroad, resulting in a follow-up period of 2.8–4.0 years for these patients. Routinely the follow-up consists of clinical visits every third month during the first year, every sixth month during the second year and annually, beginning the third year. The clinical visits included physical examination with digital rectal examination and blood studies including PSA.
Biologically no evidence of disease (bNED) was defined as PSA levels < 2 µg/l, in order to exclude PSA-bounce. Time to PSA relapse was defined as the time from radiotherapy to the third raised PSA level that was above 2 µg/l. In 1998 when this study began there had been a recent consensus meeting of ASTRO defining PSA relapse as three consecutive increased PSA measurements Citation[5]. This definition was not totally implemented in this trial since one of our laboratories did not titrate levels of PSA lower than 2 µg/l until 2000.
The side effects were assessed and recorded by the physicians according to the toxicity criteria of the USA Radiation Therapy Oncology Group (RTOG). Briefly, grade 0 corresponds to no symptoms, and grade 5 implies that the effects led to death. Grade 1–2 denotes increased amount of diarrhoea or frequency of voiding, grade 3 corresponds to severe symptoms in terms of frequency, bleeding, need of sanitary pads or requirement of surgery, grade 4 is equivalent to severe symptoms with requirement of blood transfusion or development of necrosis Citation[6]. Grades 3 to 5 refer to “major toxicities”.
The study has been approved by the ethical committee at the Karolinska Institute in Stockholm.
Treatment
In total 153 of 154 patients received neo-adjuvant hormonal therapy and external beam radiotherapy combined with 2 fractions of trans-perineal-approach HDR brachytherapy with transrectal ultrasound guidance. One patient received only two sessions of brachytherapy due to personal preferences, and developed a PSA relapse after 2.0 years. Prostate cancer cells were verified with cytology from the prostate. Since the patient violated the treatment protocol, this patient was excluded from the outcome analysis.
External beam radiotherapy
Patients received their external beam radiotherapy (EBRT) at Södersjukhuset or Radiumhemmet, Karolinska University Hospital, Stockholm. For EBRT, a computed tomography-based dose plan was established allowing a three-dimensional simulation of the radiotherapy. The planning target volume (PTV) of the EBRT included the prostate and the seminal vesicles with a 2 cm margin in all directions except posterior where the margin was reduced to 1.5 cm. The treatment units consisted of high voltage accelerators equipped with multi-leaf collimators (MLC) making the treatment a true 3D conformal therapy treatment. The patients were placed in a supine position. At Radiumhemmet the EBRT was performed with a four-field box technique. All fields were equally weighted. The dose planning system was TMS-Radix 3D® (Nucletron). At Södersjukhuset the EBRT was performed with a three-field technique, one anterior and two lateral fields. The lateral fields were weighted 50% compared to the anterior field and for the dose plans Pinnacle v. 6.2 (Philips) were used.
The target dose was 50 Gy in 2 Gy daily fractions 5 days a week. The brachytherapy was delivered after an external dose of 24 or 26 Gy, during a gap of 2 weeks. The remainder of the EBRT was delivered after the second brachytherapy session.
Brachytherapy
All patients received their brachytherapy at Radiumhemmet, Karolinska University Hospital, Stockholm. The dose-planning system (Nucletron Planning System®, Nucletron, The Netherlands) used images from transrectal ultrasound (TRUS), with images taken every 5 mm of the prostate gland. The PTV of brachytherapy included the prostate and the base of the seminal vesicles plus a 3 mm margin. The prescribed dose to the PTV was 10 Gy (×2 fractions). The HDR boost dose to the inner surface of the rectal wall was always kept below 60% of the prescribed dose of 10 Gy. The urethra was thought, during those years of treatment, to be centrally located and needles were placed so as to avoid the geometrical centre of the prostate.
The brachytherapy portion of the treatment was performed under spinal anaesthesia. The technique has previously been described in detail Citation[2]. In general 10–18 needles were used, and the duration of the procedure was usually 2 hours in total.
Statistics
The survival analyses were calculated according to Kaplan-Meyer, and the Log Rank Test was used to demonstrate differences. The Cox proportional hazards model was used in order to quantify the relationships between PSA, WHO, T-stage and PSA relapse-free survival. Student t-test was used to compare means. Analyses of count and frequency data were performed with the χ2 test. The tests were performed with the Statistica™ Release 4.1 software for Macintosh. P-values equal to or less than 0.05 were considered statistically significant.
Using the nomograms
The patients’ pre-treatment data were used in the nomogram for radiotherapy according to Kattan et al. Citation[7] and in the nomogram for surgery Citation[8]. In the nomogram for radiotherapy the highest dose-escalated total dose of 86.8 Gy was chosen for comparison.
In cases where only cytology was available, a conversion to a Gleason score was performed as follows: Cytologically well differentiated tumours (WHO grade I) = Gleason score 4, cytologically moderately differentiated tumours (WHO grade II) = Gleason score 6, and poorly differentiated tumours (WHO grade III) = Gleason score 8.
Table II. Prognostic value of WHO grade, T-stage and PSA in PSA relapse-free survival. Tested by Cox regression multivariate analyses.
The 5-year probability of PSA-free survival was defined for each patient and treatment modality. An average of the predicted 5-year probabilities was then calculated for all 154 patients using the nomograms, one set for radiotherapy and another set for surgery, to produce the third and fourth columns in .
Table III. The calculated values for the probability of 5-year PSA relapse-free survival using nomograms for surgery and dose-escalated radiotherapy are demonstrated. The patient characteristics in this study are used. The result is divided into the number of risk factors in terms of PSA, T-stage and WHO grade. The actual result from the present study is also demonstrated.
Results
Clinical outcome
In 129 living patients the median follow-up was 6.1 years (range 2.8–7.9 years). Death occurred in 24 (16%) patients, 9 (6%) of the deaths were caused by prostate cancer. The remaining 15 patients died from other causes (cardiac infarction, pancreatic neoplasm, cerebral infarction, accident) without any evidence of recurrence. Thirty-four (22%) patients developed a PSA relapse after a median follow-up time of 3.1 years (range 0.4–7.3 years). In 119 patients without a PSA relapse the median PSA value was 0.05 µg/l (range 0.02–1.9 µg/l) and 58% of the patients had a PSA value ≤ 0.05 µg/l at the latest follow-up. The majority of patients had at least one unfavourable prognostic factor. According to the number of risk factors, the observed 5-year PSA relapse-free survival was 0.97, 0.83, 0.83 and 0.51 for 0, 1, 2 and 3 risk factors, respectively. The PSA relapse-free survival according to number of risk factors is demonstrated in . Forty-seven (85%) of 55 patients with PSA < 10 µg/l at diagnosis were bNED and 72 (74%) of 97 patients with PSA ≥ 10 µg/l at diagnosis were bNED. The difference is not statistically significant using the Log Rank Test (p = 0.11). Fifty-five (69%) of the 80 patients presenting with a stage T3 tumour were bNED at their last follow-up. The relapse-free survival for patients with stage T3 tumour is demonstrated in . Taking stage T1 and T2 together, 63 (88%) of the72 patients were bNED. When patients with stage T3 were compared with patients with stage T1-2 a statistically significant difference in relapse free survival was found (Log Rank Test p = 0.003) in favour of the lower stage patients. Seventeen (61%) of 28 patients with high grade tumours (WHO grade III) were bNED at their latest follow-up. Among the 125 patients with well or moderately differentiated tumours (WHO grade I–II), 102 (72%) were bNED. Using the Log-Rank test there was a statistically significantly difference between the patients with WHO grades I-II and grade III (p = 0.005).
Figure 1. PSA relapse-free survival related to the number of risk factors. The risk factors are defined as PSA >10 µg/l, T-stage = 3 and high grade prostate cancer WHO = 3.
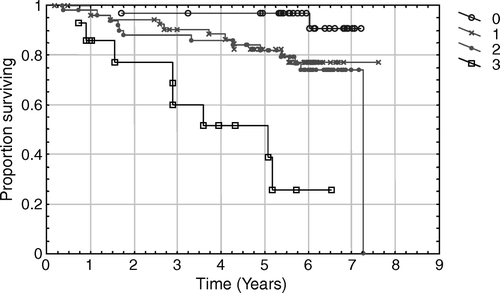
Figure 2. PSA-relapse free survival in stage T3 patients. The number of patients at risk each year is noted in the diagram.
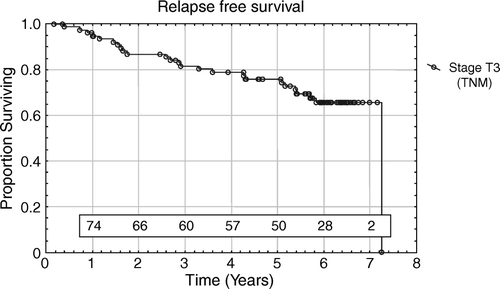
In a Cox regression univariate analyses, clinical features (PSA levels; < 10, 10–20,>20 µg/l and T-stage) and histopathologic parameters (WHO grade) yielded statistically significant prognostic information regarding bNED. In multivariate analyses only the WHO grade remained independently statistically significant ().
Side effects
Urinary tract symptoms
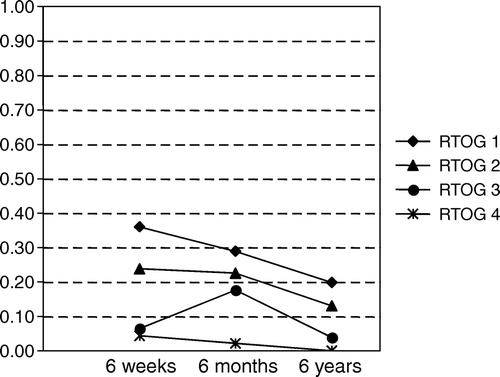
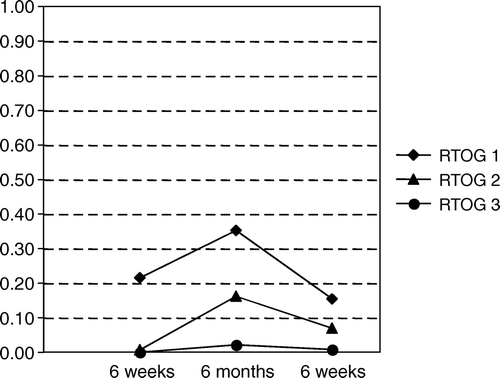
In 139, 142 and 131 patients with no relapse at 6 weeks, 6 months and last follow-up, respectively, the RTOG score for urinary tract symptoms was assessed. There was a decrease of symptoms over time and the proportion of different RTOG scores at 6 weeks, 6 months and last follow-up are summarised in .
Rectal symptoms
In 139, 142 and 130 patients with no relapse at 6 weeks, 6 months and last follow-up, respectively, the RTOG score for symptoms from the lower intestinal tract were assessed. There was a decrease of symptoms over time and the proportion of different RTOG scores at 6 weeks, 6 months and last follow-up are summarised in . Symptoms from the lower intestinal tract were less frequent than symptoms from the urinary tract.
Comparison with nomograms
The 153 patients, of whom 80 were T3, had an actual 5-year bNED survival of 84%. The predicted probability of a 5-year bNED survival was calculated using the pre-treatment data for each patient and the Kattan's nomogram for radiotherapy Citation[7] assuming the total dose of 88 Gy and the use of neoadjuvant hormonal treatment. The average probability estimated for a 5-year bNED survival following dose-escalated radiotherapy was 70%.
Regarding surgery, the probability of a 5-year bNED survival was calculated using the pre-treatment data for each patient and the nomogram for radical prostatectomy Citation[8]. The average probability for a 5-year bNED survival following surgery was predicted to be 54%. The calculated values for patients with 0-3 risk factors are demonstrated in together with the numbers from the present study.
Radiobiological consideration
Using the linear-quadratic formula and assuming that α/β is a conservative 3 Gy, the tumour effect is predicted to be equivalent to 102 Gy in 2 Gy fractions. The rectal dose, where it is possible to reduce the dose contribution from HDR brachytherapy to 60% of the prescribed dose in the prostate, will be equal to 72 Gy in 2 Gy per fractions ().
Table IV. Radiobiological calculation for tumour tissue and the rectum: F = fractions, BED = biologically effective dose, Gy3 = assuming α/β = 3, NTD = normalized dose to 2Gy per fraction.
Regardless of the α/β ratio, a comparison with a multi-centre prostate tumour dose response curves passing through the 50% 5 year relapse-free rate produced by 66 Gy with a gamma-50 slope of 2.1 brings the apparent “Intermediate Risk” result here of 88% fitting that curve at 81 Gy NTD in 2 Gy fractions, instead of at 102 Gy NTD Citation[9]. However this top end of the dose-response curve is very sensitive to uncertainties in the gamma-50 slope. If the slope were 1.4 as suggested if the "nadir + 2 µg/L” criterion Citation[10] instead of ASTRO's criterion Citation[5] was assumed, the matching dose would be close to 102 Gy NTD.
Discussion
Clinical outcome
In the effort to cure prostate cancer the radiation dose to the prostate should be equal to or exceed 70 Gy Citation[11]. When doses below 70 Gy have been used, a lower percentage of a histopathologically tumour free state have been reported Citation[12]. The use of the conformal EBRT technique has made it possible to increase the dose to 78–88 Gy Citation[13], Citation[14], without increasing the rate of side effects in the rectum or bladder. Interstitial brachytherapy provides the option of decreasing the dose to the rectum while delivering an even higher NTD-equivalent dose to the prostate. A combination of external beam conformal radiotherapy and HDR brachytherapy delivers a tumour NTD exceeding 100 Gy in 2 Gy fractions to the prostate gland (assuming the alpha/beta ratio = 3) while the dose to the anterior wall of the rectum is kept below 72 Gy provided that during the HDR boost the rectal dose is below 6.0 Gy for each brachytherapy tumour-prescribed dose of 10 Gy application (). This high dose to the tumour will provide excellent tumour response results with acceptable side effects.
Long term clinical outcomes utilizing this trans-perineal transrectal ultrasound guided technique have been published in reports from nine centres Citation[15–23]. Three of these centres have recently reported on the treatment results in more than 600 patients Citation[24]. The results are summarized in . In addition, centres have reported their experience using the Syed free-style template technique Citation[25], Citation[26]. There has been increasing experience with the use of HDR brachytherapy as a boost to external beam radiotherapy, and this has been evidenced in reports from a number of institutions of 5 years bNED survival in the range of 67–93%. These are encouraging results and our data is consistent with these earlier reports. There are surprisingly small differences in results despite the different NTD delivered to the prostate. However, no randomized trial has been conducted regarding the different fractionation schema. In addition, different selection criteria for recruiting patients, makes it difficult to compare the results.
Table V. Review of the literature. Abbreviations: 3D EBRT = Three dimensional conformal external beam radiotherapy, HDR = high dose rate brachytherapy, NTD (α/β = 3) = Normalised dose to 2 Gy per fraction and alfa/beta ratio equal to 3, RFS = relapse free survival, RTOG GU = symptoms from the urogenital tract according to the Radiation Therapy Oncology Group, RTOG GI = symptoms from lower intestinal according to RTOG.
All centres have reported the outcome data as bNED survival, however, different definitions of PSA relapse have been used, which complicates the interpretation. In an attempt to compare our results with outcome from conventional radiotherapy or surgery, we used the widely accepted nomograms according to Kattan et al. and the patient pre-treatment data from the present study. The results indicated that the outcome from surgery in this group of patients would be inferior to the outcome from combined radiotherapy. This is explained by the inclusion of a high proportion of patients with intermediate and high-risk tumours. These patients are usually not candidates for surgery due to the high risk of extra capsular extension of disease. The high rate of local control and PSA < 0.05 µg/l in 58% of the patients treated by radiotherapy, would seem to indicate that extra capsular growth is manageable using the combined external beam and HDR brachytherapy radiotherapy. The predicted differences between the results from the nomogram regarding dose escalated external beam radiotherapy and the present study were small. Since no confidence interval was presented using the nomogram, it was not possible to make any statistically significant conclusions. Furthermore, in the present study, a majority of the patients had a diagnostic fine-needle aspiration cytology performed instead of core biopsies. This introduced an uncertainty regarding the tumour grading Citation[27], Citation[28]. However, the WHO grading from cytology was converted to the lesser Gleason score in the respective interval when placed in the nomogram. For example, WHO Grade III was converted to Gleason score 8 and not 9 or 10 during calculation in the nomogram. This makes an overestimation of our results less probable.
One alternative way to report out come is to use core needle biopsy proven relapse as an end point. This has been reported in only two studies, probably because there is a risk of developing fistulae and rectal complications when performing trans-rectal biopsies of irradiated tissue. Borghede et al. reported that biopsy proven local control was found in 48 of 50 patients and only four patients demonstrated a PSA above 2 µg/l with a median follow-up of 45 months Citation[29]. Dinges et al. reported the results from 82 evaluable patients, treated with external beam radiotherapy (45 Gy in 25 fractions) combined with two sessions of brachytherapy (9 Gy a week). Of these patients, 73% had negative biopsies at 2 years follow-up and 43 patients had a PSA <1.0 µg/l after 24 months Citation[30]. In our present study, patients with PSA relapse were examined with digital rectal examination and suspected pathological findings were investigated using fine-needle aspiration cytology. No local recurrence was found.
Side effects
Different methods for the evaluation of side-effects in patients with prostate cancer have been proposed using patients' self-assessed questionnaires covering local symptoms and disease specific quality of life Citation[31] or criteria emanating from the Radiotherapy Oncology Group consensus meeting Citation[32]. There is growing knowledge that self-assessed questionnaires are more sensitive when describing the presence of side effects compared to different methods where physician report the side effects Citation[26], Citation[33], Citation[34]. However, the RTOG scores for acute and late radiation reactions have reached a consensus and a widespread use, which makes it practical when comparing different radiotherapy methods.
Differences in the rate of side effects between different centres should not in general be a result of patient selection; however, age and long-term diabetes are well-known risk factors for developing late rectal bleeding. Furthermore, there are differences in the actual delivered dose to the prostate according to the NTD. Some institutions have performed dose-escalation trials and it appears that NTD exceeding 100 Gy can be safely delivered with HDR brachytherapy boost but not using EBRT alone using delivered daily doses of 1.8 or 2 Gy fractions.
In accordance with previous studies the present trial reports a higher frequency of RTOG grade 3 symptoms from the urinary tract compared to the lower intestinal tract. The urethra is considered to be rather resistant to irradiation. However, urethral strictures, urethral necrosis, urinary incontinence have been reported Citation[30], Citation[35], Citation[36]. Galalae et al. have identified trans-urethral resection of the prostate (TUR-P) less then 6 months before irradiation as a risk factor, and they reduced the urinary tract toxicity by excluding patients with previous history of TUR-P Citation[36]. Still, the number of patients with severe symptoms in each report is limited and caution about the dose delivered to the urethra should be emphasized.
One can speculate if different numbers of needles used during HDR could impact results. A higher number of needles would facilitate a homogenous dose distribution within the prostate, but would make the implantation more complex and it would thereby be necessary to identify the urethra throughout the whole length of the prostate. In the present study no effort was made to identify the urethra in the pre-planning situation, but a surrogate urethra was used making the assumption that the urethra was centrally located. Since 2001 we have identified the urethra with the use of a urinary catheter during the pre-planning process. Preliminary outcome data evaluation has demonstrated a reduction of urinary complications. Further studies will be performed to elucidate the maximum tolerable dose to the urethra.
Conclusion
In conclusion, this present report is the experience in treatment of localized prostate cancer in a single institution with long-term follow-up. We conclude that HDR boost to the prostate combined with external beam radiotherapy and neo-adjuvant hormonal blockade, delivering an NTD over 100 Gy, can be safely given provided that precautions are taken regarding the dose to the urethra and floor of the urinary bladder. The 5-year PSA relapse-free survival rate is encouraging especially in the group of patients with intermediate risk tumours where surgery appears to produce less favourable results.
Acknowledgements
This report was supported by grants from the Cancer society in Stockholm and King Gustav V Jubilee Foundation.
References
- Cancer Incidence in Sweden (2001) 1999. Statistics-Health and Diseases 2001:4. The National Board of Health and Welfare, Centre for Epidemiology, Stockholm, Sweden, .
- Borghede G, Hedelin H, Holmang S, Johansson KA, Sernbo G, Mercke C. Irradiation of localized prostatic carcinoma with a combination of high dose rate iridium-192 brachytherapy and external beam radiotherapy with three target definitions and dose levels inside the prostate gland. Radiother Oncol 1997; 44: 245–50
- Wahlgren T, Brandberg Y, Haggarth L, Hellstrom M, Nilsson S. Health-related quality of life in men after treatment of localized prostate cancer with external beam radiotherapy combined with (192)Ir brachytherapy: A prospective study of 93 cases using the EORTC questionnaires QLQ-C30 and QLQ-PR25. Int J Radiat Oncol Biol Phys 2004; 60: 51–9
- Wahlgren T, Nilsson S, Ryberg M, Lennernas B, Brandberg Y. Combined curative radiotherapy including HDR brachytherapy and androgen deprivation in localized prostate cancer: A prospective assessment of acute and late treatment toxicity. Acta Oncol 2005; 44: 633–43
- Consensus statement: Guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 1997;37:1035–41.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6
- Kattan MW, Zelefsky MJ, Kupelian PA, Scardino PT, Fuks Z, Leibel SA. Pretreatment nomogram for predicting the outcome of three-dimensional conformal radiotherapy in prostate cancer. J Clin Oncol 2000; 18: 3352–9
- Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998; 90: 766–71
- Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low?. Int J Radiat Oncol Biol Phys 2001; 50: 1021–31
- Cheung R, Tucker SL, Lee AK, de Crevoisier R, Dong L, Kamat A, et al. Dose-response characteristics of low- and intermediate-risk prostate cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys 2005; 61: 993–1002
- Nilsson S, Norlen BJ, Widmark A. A systematic overview of radiation therapy effects in prostate cancer. Acta Oncol 2004; 43: 316–81
- Ljung G, Norberg M, Hansson H, de la Torre M, Egevad L, Holmberg L, et al. Transrectal ultrasonically-guided core biopsies in the assessment of local cure of prostatic cancer after radical external beam radiotherapy. Acta Oncol 1995; 34: 945–52
- Hanks GE, Lee WR, Hanlon AL, Hunt M, Kaplan E, Epstein BE, et al. Conformal technique dose escalation for prostate cancer: Biochemical evidence of improved cancer control with higher doses in patients with pretreatment prostate-specific antigen > or = 10 NG/ML. Int J Radiat Oncol Biol Phys 1996; 35: 861–8
- Fransson P, Löfroth P-O, Franzén L, Henriksson R, Bergström P, Widmark A. Acute side effects after dose-escalation treatment of prostate cancer using the new urethral catheter BeamCath® technique. Acta Oncol 2001; 40: 756–65
- Martinez A, Gonzalez J, Spencer W, Gustafson G, Kestin L, Kearney D, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol 2003;169:974–9; Discussion 979–80.
- Mate TP, Gottesman JE, Hatton J, Gribble M, Van Hollebeke L. High dose-rate afterloading 192 Iridium prostate brachytherapy: Feasibility report. Int J Radiat Oncol Biol Phys 1998; 41: 525–33
- Galalae RM, Loch T, Riemer B, Rzehak P, Kuchler T, Kimmig B, et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther Onkol 2004; 180: 582–9
- Pellizzon AC, Nadalin W, Salvajoli JV, Fogaroli RC, Novaes PE, Maia MA, et al. Results of high dose rate afterloading brachytherapy boost to conventional external beam radiation therapy for initial and locally advanced prostate cancer. Radiother Oncol 2003; 66: 167–72
- Hiratsuka J, Jo Y, Yoshida K, Nagase N, Fujisawa M, Imajo Y. Clinical results of combined treatment conformal high-dose-rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 59: 684–90
- Stevens MJ, Stricker PD, Saalfeld J, Brenner PC, Kooner R, O'Neill GF, et al. Treatment of localized prostate cancer using a combination of high dose rate Iridium-192 brachytherapy and external beam irradiation: initial Australian experience. Australas Radiol 2003; 47: 152–60
- Deger S, Boehmer D, Turk I, Roigas J, Wernecke KD, Wiegel T, et al. High dose rate brachytherapy of localized prostate cancer. Eur Urol 2002; 41: 420–6
- Martin T, Roddiger S, Kurek R, Dannenberg T, Eckart O, Kolotas C, et al. 3D conformal HDR brachytherapy and external beam irradiation combined with temporary androgen deprivation in the treatment of localized prostate cancer. Radiother Oncol 2004; 71: 35–41
- Astrom L, Pedersen D, Mercke C, Holmang S, Johansson KA. Long-term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother Oncol 2005;74:157–61. Epub 2004 Nov 25.
- Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 58: 1048–55
- Syed AM, Puthawala A, Austin P, Cherlow J, Perley J, Tansey L, et al. Temporary iridium-192 implant in the management of carcinoma of the prostate. Cancer 1992; 69: 2515–24
- Egawa S, Shimura S, Irie A, Kitano M, Nishiguchi I, Kuwao S, et al. Toxicity and health-related quality of life during and after high dose rate brachytherapy followed by external beam radiotherapy for prostate cancer. Jpn J Clin Oncol 2001; 31: 541–7
- Jacobs DM, Vago JF, Weiss MA. Gleason grading of prostatic adenocarcinoma on fine-needle aspiration. Diagn Cytopathol 1989; 5: 126–33
- Maksem JA, Johenning PW. Is cytology capable of adequately grading prostate carcinoma? Matched series of 50 cases comparing cytologic and histologic pattern diagnoses. Urology 1988; 31: 437–44
- Borghede G, Hedelin H, Holmang S, Johansson KA, Aldenborg F, Pettersson S, et al. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiother Oncol 1997; 44: 237–44
- Dinges S, Deger S, Koswig S, Boehmer D, Schnorr D, Wiegel T, et al. High-dose rate interstitial with external beam irradiation for localized prostate cancer – results of a prospective trial. Radiother Oncol 1998; 48: 197–202
- Litwin MS, Lubeck DP, Henning JM, Carroll PR. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: Results of the CaPSURE database. J Urol 1998; 159: 1988–92
- Watkins-Bruner D, Scott C, Lawton C, DelRowe J, Rotman M, Buswell L, et al. RTOG's first quality of life study–RTOG 90-20: A phase II trial of external beam radiation with etanidazole for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 1995; 33: 901–6
- Slevin ML, Plant H, Lynch D, Drinkwater J, Gregory WM. Who should measure quality of life, the doctor or the patient?. Br J Cancer 1988; 57: 109–12
- da Silva FC. Quality of life in prostatic carcinoma. Eur Urol 1993; 24: 113–7
- Martinez AA, Kestin LL, Stromberg JS, Gonzalez JA, Wallace M, Gustafson GS, et al. Interim report of image-guided conformal high-dose-rate brachytherapy for patients with unfavorable prostate cancer: The William Beaumont phase II dose-escalating trial. Int J Radiat Oncol Biol Phys 2000; 47: 343–52
- Galalae RM, Kovacs G, Schultze J, Loch T, Rzehak P, Wilhelm R, et al. Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 2002; 52: 81–90