Abstract
The prognostic significance of the number of lymph nodes examined in surgical specimen of colorectal cancer was determined. One thousand and twenty five patients with colorectal cancer stage II and III were included in the study. These patients underwent surgery from 1991 to 1997 and were enrolled in clinical trials to evaluate the efficacy of adjuvant 5-fluorouracil (5FU) based chemotherapy. The median number of examined lymph nodes was five. Only 13% of the patients had≥12 lymph nodes analyzed. The number of examined lymph nodes was an independent prognostic factor for overall survival in the entire group of patients with stage II and III colorectal cancer (p=0.009). Patients with a higher number of lymph nodes examined had a longer overall survival. In stage III colorectal cancer the ratio of the number of metastatic lymph nodes to the number of examined lymph nodes (lymph node ratio, LNR) was an independent prognostic factor for overall survival. A decreasing LNR was correlated with a longer overall survival (p<0.0001). Increasing age was associated with a reduction of lymph node harvest (p=0.04). Patients with rectal cancer treated with preoperative radiotherapy had a lower number of lymph nodes analyzed compared with non-radiated (p<0.001). The number of examined lymph nodes in the surgical specimen is an independent prognostic factor for overall survival in colorectal cancer. The LNR is an independent prognostic factor in stage III colorectal cancer.
Colorectal cancer is the third leading cause of cancer death in the Western world. In spite of new molecular and biochemical markers the most important prognostic factor is still the stage of disease Citation[1]. The five-year survival in colorectal cancer stage II is approximately 80% and in stage III reduced to 50%. Adjuvant chemotherapy has showed survival benefit in patients with stage III colon cancer Citation[2].
To determine the stage of disease in colorectal cancer a sufficient number of lymph nodes have to be examined. The minimum recommended number of lymph nodes necessary for adequate staging is 12 according to both the International Union Against Cancer and the American Joint Committee on Cancer. A failure to retrieve an adequate number of lymph nodes increases the risk for understaging Citation[3], Citation[4]. Understaged patients may not be given the opportunity to receive adjuvant chemotherapy. A limited number of examined nodes is a result of poor surgical- or pathological quality or both Citation[5]. It has been reported that the ratio of the number of metastatic lymph nodes to the number of examined lymph nodes (lymph node ratio, LNR) is an important prognostic factor in gastric- pancreatic- and breast cancer Citation[6–8]. Recent publications also describe the importance of LNR in colorectal cancer Citation[9], Citation[10].
The aim of this study was to define if the number of examined lymph nodes and the LNR have a prognostic value in patients after radical surgery for stage II and III colorectal cancer.
Material and methods
The study population consisted of 1 025 patients with stage II and III colorectal cancer from 50 hospitals in Sweden and two hospitals in Denmark. The patients’ demographics, tumour site, stage of disease and tumour differentiation are listed in . The patients underwent curative resection of the primary tumour from 1991 to 1997. All patients were included in prospective randomized clinical trials, randomized to surgery alone or surgery plus 5-FU based adjuvant chemotherapy. The adjuvant chemotherapy schedules included 5FU/levamisole for 12 months, 5FU/leucovorin for 4–5 months according to either a modified Mayo Clinic schedule or the Nordic schedule. Some centres also randomised patients treated with 5FU/leucovorin to +/− levamisole Citation[11]. Only patients up to the age of 76 years and with no history of other malignancies within the last five years were included. The adjuvant chemotherapy was started within 40 days after surgery in Denmark, within 70 days after surgery in Stockholm and within 49 days after surgery in the rest of Sweden. About 40% of the patients with stage II and III rectal cancer received preoperative radiotherapy with 5×5 Gy.
Table I. Patient's demographics, tumour site, stage and tumour differentiation.
Parameters of treatment, clinical outcome and date of death were obtained from the centres of Epidemiological Oncology. The quantity of resected lymph nodes and the number of lymph node metastases were obtained from the pathology reports.
Statistical methods
The endpoint for the survival analysis was overall survival (OS). To examine the relation between overall survival and stage of disease, differentiation, tumour localization, treatment, number of resected lymph nodes, number of lymph node metastases, gender and age the univariate Cox proportional hazard regression was used. Multivariate analyses were performed using Cox regression. The Kaplan-Meier method was used to create survival curves. The differences in distribution between groups were compared with the χ2 test and differences in mean with Mann-Whitney.
Results
Data from 1025 patients were eligible for this review. The median follow-up time was 5 years.
Number of analyzed lymph nodes
The median number of analyzed lymph nodes was 5.0 (range 0–32), . In stage II the median number of analyzed lymph nodes was 4.5 and in stage III 5.0. In 54 pathology reports no lymph nodes were examined.
Table II. The number of examined lymph nodes versus site of tumours.
Twelve or more lymph nodes were examined in 130 of 1 025 cases (12.7%). Twelve or more lymph nodes were retrieved in 66 of 498 (13.3%) of the patients with stage II and 64 of 527 (12.1%) in patients with stage III colorectal cancer. The total number of examined lymph nodes is stated in .
Figure 1. The number of examined lymph nodes in 1 025 patients with colorectal cancer stage II and III.
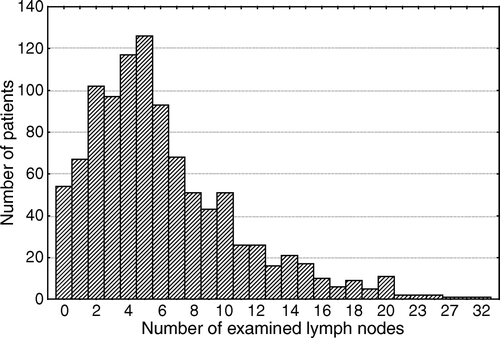
There was a significant lower number of examined lymph nodes in rectal cancer compared with colon cancer, median 4 versus 5 (p = 0.001). In patients with rectal cancer who were treated with preoperative radiotherapy (n = 113) the median number of analyzed lymph nodes was less, 4 compared to 5 lymph nodes in non-radiated patients (n = 185, p < 0.001).
Number of lymph nodes and age
The number of analyzed lymph nodes was inversely correlated to age with a lower number of analyzed lymph nodes with advancing age (p = 0.04). The inverse correlation was obvious in colon cancer patients (p = 0.03) and in rectal cancer patients treated with preoperative radiation (p = 0.02) but not in the group of rectal cancer patients who did not receive preoperative radiotherapy (n = 185, p = 0.9)
There was also a correlation between the number of examined lymph nodes and the number of lymph node metastases (p < 0.0001). The possibility to find more lymph node metastases when more lymph nodes were analyzed was apparent in both colon cancer (p < 0.0001) and rectal cancer (p < 0.0001). There was a correlation between the number of analyzed lymph nodes and stage of disease (p = 0.002).
Number of analyzed lymph nodes and prognosis
The number of analyzed lymph nodes was categorised in two groups, 0–11 versus ≥ 12.
In the entire study group the number of analyzed lymph nodes correlated with overall survival (p = 0.007), a. The 5-year survival was 64% in the group with 0–11 lymph nodes examined compared with 77% in the group with 12 or more lymph nodes examined. In the group of 54 patients without available lymph nodes to analyze, the survival rate was not different from the group of patients where 1 to 11 lymph nodes were examined (p = 0.6).
Figure 2. Kaplan-Meier plot of overall survival and the correlation to the number of examined lymph nodes. (A) Overall survival in 1025 patients with colorectal cancer stage II and III. (B) Overall survival in 498 patients with colorectal cancer stage II. (C) Overall survival in 527 patients with colorectal cancer stage III.
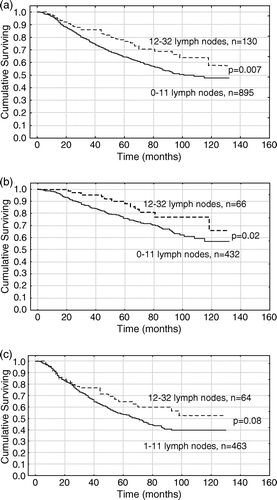
The number of analyzed lymph nodes was prognostic for patients with colon cancer (p = 0.016). In the smaller group of patients with rectal cancer the prognostic value of the number of analyzed lymph nodes did not reach a significant value (p = 0.13). When analyzing different stages we found that the number of analyzed lymph nodes had a prognostic value among patients with stage II colorectal cancer (p = 0.02), b, but did not reach a significant level in patients with stage III colorectal cancer (p = 0.08), c.
In the multivariate analysis the number of analyzed lymph nodes was of prognostic value in the entire study group of patients with colorectal cancer (p = 0.009). When analyzing the individual subgroups colon stage II, colon stage III, rectum stage II and rectum stage III respectively, we found an independent prognostic value of the number of analyzed lymph nodes only in one group, patients with colon cancer stage II (p = 0.05).
Other prognostic markers found in the entire study group beside number of analyzed lymph nodes were age, stage of disease and tumour differentiation (). Stage of disease and number of examined lymph nodes remained as independent prognostic factors for overall survival in the multivariate analysis ().
Table III. Prognostic factors in the study population of 1025 patients with colorectal cancer stage II and III.
Ratio of metastatic to examined lymph nodes in colorectal cancer stage III
Since the importance of the lymph node ratio (LNR) in patients with stage III colorectal cancer has been reported to be of prognostic value, this ratio was also calculated in this study.
The LNR was defined as a continuous variable and appeared to be a strong prognostic factor for overall survival. A low LNR correlated with a better clinical outcome (p < 0.0001). The LNR was the only independent prognostic factor in the group of patient with stage III tumours (p < 0.0001). The prognostic value of LNR was obvious in both colon (p < 0.0001) and rectal cancer (p = 0.0003).
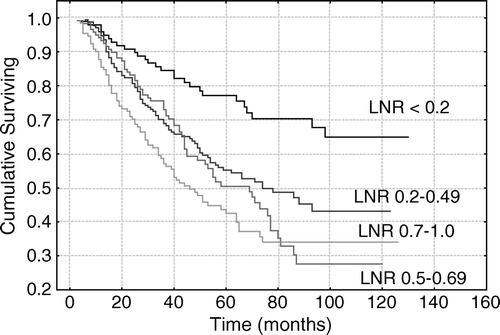
Stage III patients were also divided into four groups based on LNR. These ratios were < 0.2, 0.2 to 0.49, 0.5 to 0.69 and 0.7 to 1.0. The five year survival rates were 77%, 56%, 51% and 44%, respectively ().
Figure 3. Lymph node ratio (number of metastatic lymph nodes/number of analyzed lymph nodes) in 527 patients with stage III colorectal cancer.
There was the same distribution of LNR-groups in the two treatment arms randomized between surgery alone versus surgery and adjuvant chemotherapy.
Number of analyzed lymph nodes and survival after adjuvant chemotherapy
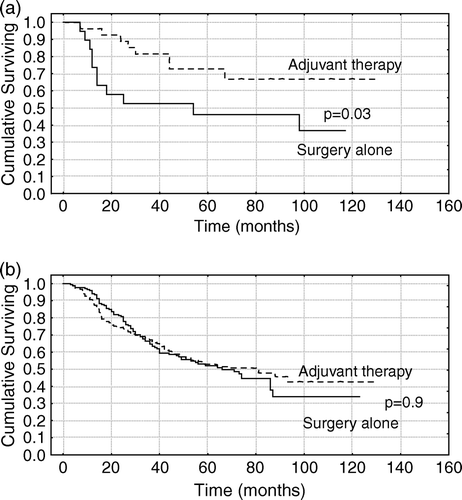
In the entire study group no difference in survival was found between the group which was treated with surgery alone compared with the group treated with adjuvant therapy. However, in the subgroup of patients with colon cancer stage III where 12 or more lymph nodes were analyzed we found longer survival among patients treated with adjuvant chemotherapy compared to surgery alone (n = 46, p = 0.03), a and b. Adjuvant treatment (p = 0.03) and the LNR (p = 0.03) were the independent prognostic factors in patients with stage III colon cancer with 12 or more lymph nodes analyzed.
Figure 4. (A) Overall survival in 46 patients with colon cancer stage III with 12 or more lymph nodes analyzed with respect to adjuvant treatment or surgery alone. (B) Overall survival in 320 patients with colon cancer stage III with one up to 11 lymph nodes analyzed with respect to adjuvant treatment or surgery alone.
Discussion
An accurate assessment of lymph node status in colorectal patients without distant metastases is essential as the presence of positive lymph nodes is used to decide whether a patient should receive adjuvant chemotherapy or not. An incorrect staging can have impact on the intensity of cancer surveillance. An inadequate lymph node sampling may lead to a failure in removing involved lymph node metastases, which consequently increases the risk of local tumour recurrence.
In accordance with other reports this study confirms that the number of analyzed lymph nodes is a prognostic factor for patients with colorectal cancer Citation[4], Citation[10], Citation[12–15]. The evaluation of high numbers of lymph nodes in stage II colorectal cancer may decrease misclassification of node-positive patients as node-negative. Poor survival among stage II patients with low number of analyzed lymph nodes may be due to stage migration. It is plausible that also interaction between tumour and host might influence the number of assessable lymph nodes. Pages et al. have found that the immune response within the tumours was associated with a less advanced pathological stage and prolonged survival Citation[16]. George et al. have analyzed data from 3 592 patients and found an association between lymphocytic infiltration into the primary tumour and survival advantage as well as more lymph nodes found Citation[15]. Reactive enlargement of lymph nodes in the mesentery may make them easier to find.
In clinical practice, there are both surgical and pathologic barriers for an accurate lymph node evaluation. Surgeons must provide the pathologists with an adequate specimen containing the tumour as well as the lymph node-bearing mesentery, which includes the main lympho- vascular supply to the bowel. The minimum number of negative lymph nodes necessary for an appropriate staging is 12 according to the American Joint Committee on Cancer and the International Union Against Cancer Citation[17], Citation[18].
There is described a variability in patient outcome in rectal cancer surgery related to case volume, with better results obtained in patients treated by high-volume surgeons Citation[19] and a strong association between surgical specialization in coloproctology and beneficial outcome Citation[20], Citation[21]. This could also have an application in the number of resected lymph nodes. There is also a variation in median number of analysed lymph nodes reported between different pathology departments Citation[9], Citation[22] which indicates that local surgical -- as well as pathology patterns may affect the adequacy of lymph node evaluation.
Patients in the present study underwent surgery between 1991 and 1997 when the impact of examining a high number of lymph nodes was still not clear. The lymph node evaluation has improved over time from a median value of four lymph nodes in 1991 to seven lymph nodes in 1997 (p = 0.003). In a study by Jestin et al. including 3 735 Swedish patients with colorectal cancer stage II and III operated between 1997 and 2002 the median number of analyzed lymph nodes was eight Citation[9].
Our study population is a selected group where patients up to the age of 76 years were included. The median age at time of surgery was 64 years which is lower than the population based median age at time for colorectal cancer surgery. However, population based reports have shown the same importance of the quality of lymph node harvesting irrespective of age Citation[9].
We found that elderly patients were more likely to have a lower number of lymph nodes evaluated than younger patients. The reason for a decreasing number of lymph nodes in elderly patients is still not clear but can be that wider lymphadenectomy is more commonly performed in the younger patients. Another hypothesis is that lymph nodes may undergo a process of involution with increasing age Citation[23]. In a report including more than 100 000 American patients with colorectal cancer Baxter et al. reported that elderly patients were less likely to receive an accurate lymph node evaluation in comparison with younger patients Citation[22]. In a study including more than 5 000 patients, Tekkis et al. found that increasing age was associated with a reduction of lymph node harvest Citation[24].
In our study approximately 40% of the patients with rectal cancer were treated with preoperative radiotherapy, (5Gy×5). We can not elucidate whether there are more advanced tumours and lower located tumours in the radiated group. However, there was the same median age and frequency of stage II and III rectal cancer in the group treated with surgery alone compared with the group treated with radiotherapy after operation and the survival rate was similar in the two groups. We have revealed that the number of lymph nodes harvested from rectal cancer patients who were treated with radiotherapy preoperatively was significantly lower compared with the group who had surgery without preoperative radiotherapy which also has been reported by others Citation[24–26]. Ionizing radiotherapy has a substantial and prolonged effect and is associated with lymphocyte depletion in normal lymph nodes, with atrophy and fibrosis of the stroma. This might result in a decrease in lymph node size or can even make the lymph node unrecognizable Citation[27]. Lymph node staging in rectal cancer patients who are treated with preoperative radiotherapy must be interpreted with caution.
We found that the number of lymph nodes was a prognostic factor in stage II colorectal cancer but the prognostic value did not reach significant level in stage III colorectal cancer. However, in the group of N1-tumours with 1–3 lymph node metastases we found a prognostic value in the number of analyzed lymph nodes (p = 0.03) but not among N2 tumours. There is a potential risk of incorrect staged N1 tumours with a shorter overall survival in the N1 stage group when few lymph nodes are analyzed.
There are studies including a higher number of patients reporting a prognostic value of the number of analyzed lymph nodes also in stage III colorectal cancer Citation[9], Citation[15].
We have found that the LNR in both colon and rectal cancer is a prognostic factor which is in accordance with other reports Citation[9], Citation[10]. The LNR was a prognostic factor in the N1 group with 1–3 metastases but not in the group of 114 patients with N2 tumours.
However others who have studied material including more patients with N2 tumours have presented changes in survival based on LNR in both N1 and N2 tumours Citation[10]. The LNR is a reflection of the number of positive nodes and the total number of evaluated lymph nodes which have already been shown to be important prognostic factors for colorectal cancer Citation[12], Citation[14]. With increasing number of metastatic lymph nodes and unchanged total number of lymph nodes the LNR will increase. An increasing LNR correlates to a decreased survival.
Among the 2 211 patients included in the Nordic adjuvant studies there was a 7% absolute benefit in overall survival for patients with stage III colon cancer receiving adjuvant 5-FU based chemotherapy but without reaching significant level Citation[11]. In the present retrospective analysis including a subgroup of 1 025 patients from the Nordic trials there was no difference in survival between the group treated with surgery alone compared with the group treated with adjuvant chemotherapy after surgery. When dividing the study population into subgroups we found a longer overall survival in the group of patients with colon cancer stage III with 12 or more analyzed nodes who was treated with chemotherapy compared with the group treated with surgery alone (p = 0.03, n = 46). However, this finding should be interpreted with caution given that the number of patients is too small to conclude whether the quality of node harvesting has any impact on the benefit of adjuvant chemotherapy or not. The extent of benefit from adjuvant chemotherapy is reported to relate to tumour grade, invasion and nodal involvement and patients at highest risk of recurrence are those who seem to gain most from adjuvant therapy Citation[2].
Conclusion
In the presented material of patients who underwent surgery from 1991 to 1997 a majority had an inadequate lymph node evaluation according to the American Joint Committee on Cancer and the International Union Against Cancer Citation[17], Citation[18] and only 13% of the patients had 12 or more nodes examined. The number of examined nodes was an independent prognostic factor for overall survival in the entire group and in the subgroup of patients with stage II colorectal cancer. In the group of patients with stage III colorectal cancer the prognostic value did not reach a significant value (p = 0.08). However, the ratio between the number of analyzed lymph node metastases and the number of examined lymph nodes (LNR) was an independent prognostic factor in stage III colon cancer and stage III rectal cancer. We have confirmed that preoperative radiotherapy of rectal cancer is associated with a lower number of examined lymph nodes compared with non-radiated rectal cancer patients. In the studied patient group there was a correlation between higher age and lower number of examined lymph nodes. It is very important to improve the lymph node retrieval as it has great impact on the postoperative treatment and prognosis. Every effort to improve quality of care in this field will result in better clinical outcome for patients with colorectal cancer.
Acknowledgements
We would like to thank the Oncology Center in Umeå, Uppsala, Stockholm, Linköping, Göteborg, Lund and Denmark. We thank Bo Nilsson and Göran Granat for help with statistical analysis. Financial support was obtained from the Swedish Cancer Society, the Swedish Society of Medicine (Bengt Ihre Foundation), the Nordic Cancer Union and Gustav V's Jubilee Foundation. None of the authors have declared any conflicts of interest.
References
- Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000; 88: 1739–57
- Benson AB, 3rd. New approaches to the adjuvant therapy of colon cancer. Oncologist 2006; 11: 973–80
- Joseph NE, Sigurdson ER, Hanlon AL, Wang H, Mayer RJ, MacDonald JS, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol 2003; 10: 213–8
- Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003; 10: 65–71
- Bilchik A. More (nodes) + more (analysis) = less (mortality): Challenging the therapeutic equation for early-stage colon cancer. Ann Surg Oncol 2003; 10: 203–5
- Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 2002; 9: 775–84
- Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2004;70:235–40; Discussion 240.
- Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol 2006; 24: 2910–6
- Jestin P, Pahlman L, Glimelius B, Gunnarsson U. Cancer staging and survival in colon cancer is dependent on the quality of the pathologists' specimen examination. Eur J Cancer 2005; 41: 2071–8
- Berger AC, Sigurdson ER, Levoyer T, Hanlon A, Mayer RJ, Macdonad JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005; 23: 8706–12
- Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, et al. Adjuvant chemotherapy in colorectal cancer: A joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol 2005; 44: 904–12
- Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J Clin Oncol 2003; 21: 2912–9
- Prandi M, Lionetto R, Bini A, Francioni G, Accarpio G, Anfossi A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: Results of a secondary analysis of a large scale adjuvant trial. Ann Surg 2002; 235: 458–63
- Tepper JE, O'Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB 3rd, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001; 19: 157–63
- George S, Primrose J, Talbot R, Smith J, Mullee M, Bailey D, et al. Will Rogers revisited: Prospective observational study of survival of 3 592 patients with colorectal cancer according to number of nodes examined by pathologists. Br J Cancer 2006; 95: 841–7
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–66
- Sobin LH, Greene FL. TNM classification: Clarification of number of regional lymph nodes for pNo. Cancer 2001; 92: 452
- Wittekind CH WG. Colon and rectum. In: TNM-Classification of malignant tumors. New York, NY: Springer; 1997. p 64–7.
- Martling A, Cedermark B, Johansson H, Rutqvist LE, Holm T. The surgeon as a prognostic factor after the introduction of total mesorectal excision in the treatment of rectal cancer. Br J Surg 2002; 89: 1008–13
- Holm T, Johansson H, Cedermark B, Ekelund G, Rutqvist LE. Influence of hospital- and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg 1997; 84: 657–63
- Smith JA, King PM, Lane RH, Thompson MR. Evidence of the effect of'specialization' on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg 2003; 90: 583–92
- Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: A population-based study. J Natl Cancer Inst 2005; 97: 219–25
- Schmucker DL, Owen RL, Outenreath R, Thoreux K. Basis for the age-related decline in intestinal mucosal immunity. Clin Dev Immunol 2003; 10: 167–72
- Tekkis PP, Smith JJ, Heriot AG, Darzi AW, Thompson MR, Stamatakis JD. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis Colon Rectum 2006.
- Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, van de Velde CJ, Leer JW, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol 2001; 19: 1976–84
- Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: A population-based analysis. Int J Radiat Oncol Biol Phys 2005; 61: 426–31
- Fajardo LF. Effects of ionizing radiation on lymph nodes. A review. Front Radiat Ther Oncol 1994; 28: 37–45
