Abstract
Despite a decline in its incidence in the Western world, gastric cancer (GC) remains the fourth most frequent cancer diagnosis worldwide and is, after lung cancer, the second leading cause of death from a malignant disease globally. Based on the published literature, treatment guidelines and reports from international meetings, we here review the current treatment options for GC and discuss insights and perspectives from the latest clinical studies. The management of GC in the early stages of the disease is based on an optimal surgical resection of the primary tumor and the regional lymph nodes. However, less than one third of patients have a resectable disease at diagnosis and among those operated, more than half are not cured by surgery alone, due to a high rate of relapse. Thus, for the majority of patients, systemic cytotoxic therapy, and sometimes radiotherapy, is a treatment option both as an adjunct to surgery and in the palliative setting. Adjuvant chemotherapy offers only a marginal benefit and has not become a standard of care in the West. In North America, adjuvant chemoradiation is broadly used, shown to significantly improve overall survival, albeit with the cost of high toxicity. Furthermore, a recently reported study from the United Kingdom demonstrated a significant disease-free and survival benefit by the use of perioperative combination chemotherapy. Several chemotherapeutic agents have been tested as a palliative therapy in advanced GC including 5- fluorouracil (5-FU), oral pyrimidines, platinum derivatives, anthracyclines, taxanes and camptothecans. It is now accepted that chemotherapy is better than best supportive care only and that 5-FU based combinations are more effective than monotherapy. However, the response rates have generally been moderate and there is no consensus on the optimal combination of cytotoxic agents and the potential role of more recently developed “targeted therapies”.
Historically, gastric adenocarcinoma (GC) has been one of the most prevalent malignancies worldwide, but its incidence is decreasing since the beginning of the 20th century, mainly in the Western world. However, from a global perspective, GC is still an important health problem with almost one million new cases and 700 000 deaths annually Citation[1]. The incidence is highest in East Asia, Eastern Europe, and South America. Presently, GC accounts for 2.6% of all new cancers in Sweden Citation[2].
The cornerstone in the treatment of GC is surgical resection of the primary tumor. Tumors confined to the mucosa and submucosa of the stomach are cured by gastrectomy in more than 90% of the cases Citation[3]. However, when the tumor extends through the gastric wall and/or metastasizes to the regional lymph nodes, the prognosis after surgery alone is dismal with a 5-year survival of less than 30% Citation[4], indicating the need for effective adjunctive therapies for these patients. In the Western world, more than two thirds of the new cases with GC are diagnosed when the tumor is irresectable or metastatic. In these patients, as well as in cases with disease recurrence after initial surgical treatment, palliative therapy is of importance for controlling tumor related symptoms, improving quality of life Citation[5], Citation[6], and prolonging survival Citation[5–8]. To date, the response rates of chemotherapy in patients with advanced GC have been moderate and the median survival in this patient group is still less than one year. Thus, there is a great interest in newer cytotoxic agents, “targeted therapies”, and novel combinations, which are currently tested in clinical trials, in an effort to define a new gold standard of treatment for this stage of the disease.
In this article, we review the current treatment options for patients with GC including surgery, adjuvant and palliative therapies and discuss insights and perspectives from the latest clinical studies and from important ongoing projects.
Methods
A PubMed database search of all randomized phase III studies and selected phase II studies of gastric cancer published until October 2006 was performed. Relevant reports from the latest American Society of Clinical Oncology (ASCO) Annual Meetings and Gastrointestinal Symposia were also included. The following keywords were used in the searches: “gastric/stomach carcinoma/cancer”, “surgery”, “chemotherapy”, “radiotherapy”, “targeted therapy”. Furthermore, national and international treatment guidelines as well as review papers by world leaders in the field were considered.
Results
Surgery
The outcome of surgical treatment of GC, in terms of long-term survival, has in essence been disappointingly stable over the recent decades. Within this time frame, clinical research activities have, however, been able to elucidate the mechanisms behind adverse effects of the surgical interventions and thereby define options to facilitate the functional outcome Citation[9]. Moreover, data have been presented to allow clinicians to choose between surgical options with an evidence-based conceptual approach and to open up for a tailored therapeutic approach based on patient and tumor specific characteristics. Gastric cancer resections have traditionally been looked upon as procedures that can and shall be practised in every hospital irrespective of the level of specialisation Citation[10]. This contrasts to the situation for oesophageal cancer surgery; since the established differences in postoperative morbidity and mortality have been less clear for GC surgery. Despite corresponding circumstances there are three pivotal factors that in the future eventually will lead to a centralisation of these cases to specialised centres. First, the number of cases will continue to decrease due to the steadily declining incidence of GC. Moreover, all studies have as yet in fact revealed a quality gain by having the operations done in high volume centres and, lastly, most surgeons will have a suboptimal experience from elective gastric surgery due to the disappearance of surgery for benign gastric diseases Citation[10].
Two important aspects relevant to the surgical practise in GC have been elucidated within the framework of randomised clinical trials (RCTs). The long lasting question of whether total gastrectomy always should be performed or whether subtotal resections can be recommended, based on the location and histomorphology of the primary tumor, has now been settled. The latter strategy should be looked upon as the “standard of care” which, however, means that in patients with diffuse cancer virtually always a total gastrectomy is mandated to guarantee an R0 resection Citation[11–13]. Parallel with these scientific endeavours RCTs were conducted to assess the true value of extended lymphadenectomy (D2 vs. D1 dissection). Although grade II evidence would support the value of extended lymphadenectomy (D2) subsequent RCTs have been unable to reveal an advantage of this surgical approach when overall long-term survival is concerned Citation[14–18]. On the contrary these trials, as well as subsequent studies, revealed an enhanced complication risk if splenectomy and/or pancreatectomy were added to this extended procedure. These data should therefore form the basis for the standard of surgical care, which means that splenectomy and/or concomitant resection of the pancreatic tail should be avoided if possible Citation[19].
There are, however, other issues relevant to the question of the most efficient way of performing surgery in GC. Local tumour control and clearance are pivotal and at the time point of surgery it is the unique opportunity to achieve this. Thereby an optimal environment has been established for adjuvant therapies to be as effective as possible. A similar standardised surgical strategy offers also tissue specimens that allow an adequate staging and classification of the disease. This is of course of outmost importance when RCTs are embarked upon.
Reconstruction after a total gastrectomy has to be completed by the use of a 60 – 70 cm long Roux-en-Y oesophago-jejunostomy. The postoperative rehabilitation after corresponding operations is characterised by weight loss due to inadequate calorie intake and/or enhanced losses due to diarrhoea. A number of RCTs have consistently demonstrated that construction of a gastric reservoir (pouch) is preferable and can effectively counteract some of the side effects referred to above Citation[20–26]. One of the most important consequences of such a reconstructive strategy is an improved quality of life. Another area for future development, in order to maintain as much of the normal gastrointestinal functions as possible, is to master the concept of vagal sparing resections Citation[27]. In those situations, it is vital to save the function of the lower oesophageal as well as the pyloric sphincters. It is crucial to endorse this concept based on the skill to also concomitantly carry out a local extended lymphadenectomy. Thereby the tumor staging and local disease control objectives are secured. With the advent of a more frequent use of minimal invasive surgical techniques in the management of early GC, the role of sentinel node navigated surgery has to be further explored Citation[28].
Adjuvant therapies
The rates of recurrence after an apparently radical surgical resection are high irrespective of the method used; hence adjunctive therapies for GC have been widely explored during the past decades. The efficacy of chemotherapy and radiotherapy as an adjunct to surgery has been tested alone or in combination, both pre- and postoperatively.
Adjuvant, postoperative chemotherapy
The value of adjuvant, postoperative chemotherapy in GC has been debated during the last two decades as none of the about twenty performed RCTs has shown a survival advantage compared to surgery only in a Western population Citation[29]. Since 1993, six meta-analyses have been published indicating a small but probably clinically insignificant benefit for postoperative chemotherapy compared to the control groups Citation[29–34]. It should be noted though that a great heterogeneity in chemotherapy regimens and study size was evident in these pooled analyses and that newer chemotherapeutic regimens, that have shown high response rates in metastatic GC, have not been fully evaluated in the adjuvant setting. However to date, postoperative chemotherapy alone is not accepted as a standard adjunct to surgery in most Western countries.
Adjuvant, postoperative radiochemotherapy
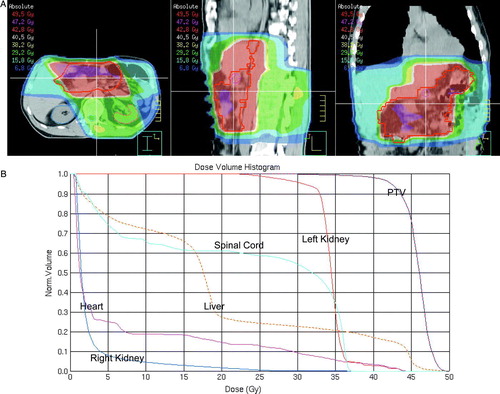
The combination of adjuvant chemotherapy and radiotherapy is routinely used in the United States (US) after the publication of the Intergroup 0116 trial (n = 556) Citation[35]. This study demonstrated a clinically significant increase in the 3 year overall survival rate for patients with non-metastatic, Stage ≥ 2 GC receiving postoperative treatment with 5-fluoruracil (FU)/leucovorin and radiotherapy after radical surgery compared with the control, i.e. 50% vs. 41%. However, due to concerns about the toxicity of the therapy and criticism regarding the type of surgery used in this trial, i.e. in more than 50% cases less than D1-dissections, adjuvant chemoradiation has not been widely adopted in Europe. The used radiotherapy planning method Citation[36], viz. 2 dimensional (2 D) planning based on radiographs, is furthermore considered outdated and it has since decades been replaced by 3 D planning based on computed tomography (CT) scans. Target volume definition is difficult in the postoperative setting and must include evaluation of pre- and post-operative CT scans of the abdomen and careful review of the surgical findings/method and pathology report Citation[37]. Examples of an individualized 3 D dose plan and dose volume histograms for the target volumes and organs at risk are shown in A and B, respectively, in a patient who had undergone a distal subtotal gastrectomy.
Figure 1. Adjuvant radiotherapy after distal subtotal gastrectomy (A) 3D dose plan. The red line depicts the planning target volume (PTV) and the red area 95% of the prescribed dose (B) Dose volume histograms for the PTV and organs at risk.
In analogy to the Intergroup 0116 trial, a North American, phase II study of preoperative chemoradiation, viz. RTOG 9904, was recently published Citation[38]. The results are promising, with pathologic complete responses exceeding 20% and a planned randomized phase III trial may elucidate whether neoadjuvant chemoradiation will have a place in the curative treatment of GC.
Perioperative chemotherapy
Until recently, preoperative therapy was not routinely applied in GC, although the results of phase II trials of neoadjuvant chemotherapy were promising Citation[39–42]. The use of neoadjuvant therapy for GC has come into focus after the publication of the MAGIC study, conducted by the British Medical Research Council (MRC) Citation[43]. In this phase III trial (n = 503), patients with resectable, non-metastatic Stage ≥2 adenocarcinoma of the stomach were randomized to receive either perioperative chemotherapy or surgery only. Chemotherapy consisted of three preoperative and three postoperative cycles of epirubicin, cisplatin, and infused 5-FU (ECF). The results show a statistically significant improvement in disease-free survival and overall survival (5 year survival 36% vs. 23%) as well as decreased tumor size and stage by the use of perioperative ECF treatment. At last years’ Annual Meeting of the American Society of Clinical Oncology (ASCO), the results from a similarly designed French study (n = 224) were reported and clinically significant improvement in 5 year disease free survival was found in patients randomized to perioperative cisplatin-based combination chemotherapy compared to the control group, which underwent direct surgery (34% vs. 17%) Citation[44]. Data on overall survival from the latter trial are expected.
Palliative, systemic therapies
In selected, fit patients with advanced GC, systemic chemotherapy offers moderate but clinically significant advantages Citation[5–8] in terms of increased survival, i.e. of about 6 months, symptoms control, and quality of life Citation[5], Citation[6], compared to best supportive care only. Currently, the clinical research in palliative therapy focuses on platinum compounds, the topoisomerase I inhibitor irinotecan, the taxane docetaxel, the oral pyrimidines capecitabine and S-1, and “targeted therapies.”
Platinum derivatives
Cisplatinum has been used extensively as an integral part of combination chemotherapy regimens in patients with advanced GC. In the US, bolus cisplatin has most commonly been combined with a 5 day 5-FU infusion (CF). In Great Britain, epirubicin has also been included and evaluated in the ECF-regimen Citation[45], Citation[46]. A recently performed meta-analysis suggests that the addition of an anthracycline, e.g. epirubicin, to cisplatin based regimens independently improves survival for patients with metastatic GC Citation[47].
The first results from a recently finished British study on advanced GC, the REAL 2 trial, were reported at the 2006 Annual Meeting of ASCO Citation[48]. This non-inferiority, randomized phase III trial indicates that oxaliplatin may replace cisplatin and capecitabine may replace 5-FU in the ECF regimen without significant differences either in response rates or in survival.
Irinotecan
Another evolving agent in the treatment of GC is the topoisomerase I inhibitor irinotecan. The efficacy of irinotecan in GC has been demonstrated in several phase II studies, both as monotherapy and in combination regimens Citation[49–53]. Results from a phase III trial (n = 337) that compared a combination of irinotecan and infusion 5-FU + leucovorin (IFL) to CF indicate a trend toward longer time to progression and better overall survival as well as better tolerability for the irinotecan-based regimen Citation[54].
Docetaxel
The recently presented V325-study, a randomized phase III trial (n = 457), demonstrated that the addition of docetaxel to CF (DCF) was significantly superior to the reference combination (CF), improving response rate, time to progression, overall survival, and quality of life for patients with advanced GC Citation[55]. Of note, a Swiss-Italian randomized phase II study (n = 121) compared ECF, with docetaxel containing regimens, either DCF or docetaxel-cisplatin (DC) Citation[56]. The preliminary results suggest a higher activity of DCF in terms of response rate and time to progression with the cost of more frequent episodes of neutropenia compared to ECF.
Oral pyrimidines
Capecitabine is an oral, inactive 5-FU prodrug. It is extensively absorbed by the gut mucosa and its final conversion to 5-FU in tumor cells is facilitated by a higher expression of the enzyme thymidine phosphorylase than in normal tissues. Capecitabine can replace infused 5-FU in the ECF regimen according to the previously described results of the REAL 2 study Citation[48]. Kang and co-workers have, furthermore, reported that capecitabine can replace infused 5-FU in the CF-regimen with similar efficacy and safety in patients with advanced GC (n = 316) Citation[57].
The combination of irinotecan and capacitabine has been tested in one phase II trial in patients with advanced GC Citation[58]. Data from studies in patients with metastatic colorectal cancer have indicated an overlapping toxicity problem with severe diarrhea, necessitating dose reductions Citation[59]. Presently, there are no results from phase III trials comparing the irinotecan/capecitabine combination with any of the standard regimens, i.e. ECF or CF.
Another oral pyrimidine, viz. S-1, has been routinely used in Japan both as a single agent and in combination regimens with promising response rates. However, due to considerable pharmacokinetic differences when used in Caucasians, S-1 was only recently introduced in the West. Phase I and II studies have been completed for the combination regimen of cisplatinum and S-1 and they indicate a potential activity and favorable toxicity profile for S-1 Citation[60], Citation[61]. The regimen of S-1 (25 mg/m2 orally b.i.d. on days 1–21) plus cisplatinum (75 mg/m2 i.v. on day 1) every 28 days is compared with a standard cisplatinum + 5-FU regimen in the ongoing phase III FLAGS trial.
Targeted therapy
Novel drugs including epidermal growth factor receptor (EGFR) targeted therapies, matrix metalloproteinase (MMP) inhibitors, and inhibitors of angiogenesis are presently tested in advanced GC.
Molecular studies indicate that EGFR is over-expressed in a proportion of GC and is a negative prognostic factor, hence theoretically, an attractive therapeutic target Citation[62]. Clinically developed drugs targeting EGFR include the monoclonal antibody cetuximab and the tyrosine kinase inhibitors erlotinib and gefitinib. Cetuximab combined with chemotherapy or radiotherapy has shown activity in several tumor types including colorectal, lung and head and neck cancers. Phase II studies in GC are ongoing but no results about its efficacy are available yet. Gefitinib has been evaluated in a small phase II trial of 75 patients with advanced GC Citation[63]. The drug was well tolerated but the response rate was modest, 18.3%. Similarly, erlotinib was tested in a study of 70 patients with either esophageal cancer (n = 46) or GC (n = 24) but none of the patients with GC responded to the treatment Citation[64].
Matrix metalloproteinases are a family of enzymes that are important for tumor invasion and metastasis and were thus early recognized as targets for cancer therapy. The orally available MMP inhibitor marimastat demonstrated a trend to survival benefit in a small phase III study of GC and was proposed as a maintenance therapy for patients with advanced GC Citation[65]. However, no other studies have been reported and marimastat has not become routinely available for patients with GC.
The principle of inhibiting tumor growth by blocking the formation of new blood vessels is now introduced into clinical practice. Bevacizumab, a monoclonal antibody that binds specifically to the vascular endothelial growth factor (VEGF), has recently been approved for the treatment of metastatic colorectal cancer and is currently evaluated for numerous other indications. In GC, concerns have been raised that the risks for bleeding and thromboembolic events, inherent to the disease, may be aggravated by bevacizumab. An ongoing phase II study of weekly docetaxel and bi-weekly bevacizumab for advanced GC (AvaTax) reported that after the inclusion of 20 patients, three cases of gastrointestinal hemorrhage and two of arterial thrombosis were observed Citation[64]. The comparison of two parallel phase II studies of irinotecan–based chemotherapy for GC, one with bevacizumab (National Cancer Institute (NCI) protocol 6447) and one without (NCI protocol 5917) indicated that there is no significant increase in the rate of thromboembolic events by the addition of bevacizumab Citation[66]. The preliminary results from these trials suggest that bevacizmab is active against GC, but extended data from RCTs in a larger number of patients are needed before conclusions can been drawn.
Discussion
The cornerstone in the treatment of localized GC is an optimal and standardized resection of the primary tumor and the regional lymph nodes. Well-trained surgeons in high-volume centers should perform the surgical procedure to minimize postoperative morbidity and mortality. The quality of surgery should be continuously monitored through population-based national quality registries. Such a registry has been successfully started in Sweden in 2006, viz. the NREV-registry. The optimal degree of lymph node dissection is still debated but the standard procedure should be more than a D1-dissection for staging reasons. D-2 dissections, without splenectomy and/or pancreatic tail resection, can be recommended in younger and fit patients to increase the accuracy of the N-staging to identify patients who incorrectly have been understaged preoperatively, i.e. Stage < 2, and who may benefit from “rescue” adjuvant radiochemotherapy. Standard additional therapy in operable patients with Stage ≥2, non-metastatic GC should primarily consist of platinum-based perioperative chemotherapy Citation[43]. Recent data support the use of EOX (epirubicin, oxaliplatin, capecitabine) in this setting Citation[48]. The adoption of the latter strategy may increase the proportion of patients who can complete three courses of postoperative therapy compared to the original MAGIC-results, i.e.<50% Citation[43], and potentially further increase the effectiveness of perioperative treatment.
New trials on adjuvant therapy in GC are ongoing or planned. In the USA, RTOG is testing whether a postoperative radiochemotherapy regimen which includes ECF is better than the original schedule which only included bolus 5-FU. This study has presently included about 300 of the planned total of 600 cases. In Great Britain, the MRC is planning to evaluate an additional therapeutic effect of bevacizumab to the MAGIC concept. In the Netherlands, plans are underway to evaluate the addition of postoperative radiochemotherapy to the MAGIC strategy. Furthermore, the EORTC/PETACC group may launch a phase II trial to test the feasibility of preoperative radiochemotherapy, which incorporates the use of some of the newer cytotoxic agents. As mentioned above, the definition of target volumes in gastric irradiation is challenging. Work is currently underway to facilitate this process by the use of 3D atlases depicting the location of suitable target structures based on large Japanese surgical series Citation[67], Citation[68]. Two papers have also been published on this topic which can further assist the inexperienced, non-specialized radiotherapist with the task of performing individualized radiotherapy planning Citation[36], Citation[37].
Presently, there are a number of acceptable chemotherapy regimens that can be recommended in the palliative stage of GC in addition to best supportive care (summarized in ). A careful evaluation of the patient's performance status and the different toxicity profiles of the regimens should be performed before start of therapy. In a global perspective, both CF and ECF are still acceptable choices in the treatment of advanced GC. The recent meta-analysis of the Cochrane Institute concludes that epirubicin and cisplatin contribute independently to the effectiveness of combination chemotherapy in this setting, which supports the incorporation of epirubicin in a standard regimen. Preliminary results of the REAL-2 trial indicated that the EOX-arm was superior to the other three study arms in terms of survival Citation[48]. This finding, the convenience of oral replacement of infused 5-FU by capecitabine and the often more favorable toxicity profile of oxaliplatin compared to cisplatin, support the introduction of the EOX-regimen as a new reference chemotherapy combination that be can be used broadly. In younger and fit patients, the new DCF-regimen, which includes the newer agent docetaxel, may be an alternative to CF. Many European physicians, however, consider high-dose cisplatin combinations, i.e. CF and DCF, too strenuous for patients treated for palliation due to their known severe toxicities. Since the first report of irinotecan's high activity in advanced GC, as compared to the previously used etoposide based ELF-regimen Citation[51], a great interest in its use has been seen in Sweden. Presently, a modified ILF regimen is considered the reference regimen in the ongoing Swedish randomized phase II study, viz. the GATAC-study, which tests if docetaxel can replace irinotecan in an infusion 5-FU based combination regimen.
Table I. Phase III studies supporting the use of the currently recommended combination chemotherapy regimens for the palliative treatment of gastric cancer.
The presently available data do not support the routine use of targeted therapies in GC. The strategies of blocking the EGFR-initiated pathway and inhibiting angiogenesis appear promising and RCTs of the drugs that have showed some anti-tumoral activity in early clinical studies are ongoing. Nevertheless, more potent agents, better combinations with cytotoxic drugs and most importantly a biologically rational selection of the patients who will benefit from the treatment may be required for these novel therapies to demonstrate a clinically significant effect. Furthermore, more clinical experience is required to fully characterize the potential risks linked to the use of these agents in general and in GC in particular.
In conclusion, an optimal surgical resection of the primary tumor remains the therapeutic cornerstone in patients with localized, non-metastatic GC. Perioperative cisplatin-based chemotherapy should, however, be considered in fit patients to reduce recurrences and increase survival. In patients who have been “understaged” preoperatively, postoperative radiochemotherapy may be recommended in selected cases. Presently, there are several acceptable treatment regimens for patients with metastatic GC, as a number of newer drugs have shown efficacy in this disease. Several targeted therapies are currently being evaluated in GC and preliminary data suggest that these compounds may in the future be incorporated in the therapeutic armamentarium both in the palliative and adjuvant settings.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108
- The National Board of Health and Welfare: Cancer Incidence in Sweden (2003) 2001. Stockholm, .
- Karpeh M, Kelsen D, Tepper J. Cancer – Principles & practice of oncology, V Cancer of the stomach. De Vita, S Hellman, S Rosenberg. Lippincott Williams & Wilkins, Philadelphia 2000; 1092–126
- DeVita VTJ, Hellman S, Rosenberg SA. Cancer: Principles and practice of oncology7th ed. Lippincott Williams & Wilkins, Philadelphia 2005
- Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. Br Med J 1993; 306: 752–5
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997; 8: 163–8
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993; 72: 37–41
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995; 71: 587–91
- Lehnert KB. Techniques of reconstruction after total gastrectomy for cancer. Br J Surg 2004; 91: 528–39
- McCulloch P. Should general surgeons treat gastric carcinoma? An audit of practice and results, 1980–1985. Br J Surg 1994; 81: 417–20
- Gouzi JL, Huguier M, Fagniez PL, Launois B, Flamant Y, Lacaine F, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg 1989; 209: 162–6
- Robertson CS, Chung SC, Woods SD, Griffin SM, Raimes SA, Lau JT, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg 1994; 220: 176–82
- Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: Five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 1999; 230: 170–8
- Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999; 340: 908–14
- Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999; 79: 1522–30
- Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004; 22: 2069–77
- McCulloch, P, Nita, ME, Kazi, H, Gama-Rodrigues, J. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev 2004: CD001964.
- Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT, Whang-Peng J. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg 2004; 91: 283–7
- Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 1988; 75: 110–2
- Liedman B, Bosaeus I, Hugosson I, Lundell L. Long-term beneficial effects of a gastric reservoir on weight control after total gastrectomy: A study of potential mechanisms. Br J Surg 1998; 85: 542–7
- Svedlund J, Sullivan M, Liedman B, Lundell L. Long term consequences of gastrectomy for patient's quality of life: The impact of reconstructive techniques. Am J Gastroenterol 1999; 94: 438–45
- Fuchs, KH, Thiede, A, Engemann, R, Deltz, E, Stremme, O, Hamelmann, H. Reconstruction of the food passage after total gastrectomy: Randomized trial. World J Surg 1995;19:698–705; Discussion –6.
- Schwarz, A, Buchler, M, Usinger, K, Rieger, H, Glasbrenner, B, Friess, H, et al. Importance of the duodenal passage and pouch volume after total gastrectomy and reconstruction with the Ulm pouch: Prospective randomized clinical study. World J Surg 1996;20:60–6; Discussion 6–7.
- Nakane Y, Okumura S, Akehira K, Okamura S, Boku T, Okusa T, et al. Jejunal pouch reconstruction after total gastrectomy for cancer. A randomized controlled trial. Ann Surg 1995; 222: 27–35
- Iivonen MK, Mattila JJ, Nordback IH, Matikainen MJ. Long-term follow-up of patients with jejunal pouch reconstruction after total gastrectomy. A randomized prospective study. Scand J Gastroenterol 2000; 35: 679–85
- Liedman B, Andersson H, Berglund B, Bosaeus I, Hugosson I, Olbe L, et al. Food intake after gastrectomy for gastric carcinoma: The role of a gastric reservoir. Br J Surg 1996; 83: 1138–43
- Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: Prospective randomized study. World J Surg 2005; 29: 1592–9
- Uenosono Y, Natsugoe S, Ehi K, Arigami T, Hokita S, Aikou T. Detection of sentinel nodes and micrometastases using radioisotope navigation and immunohistochemistry in patients with gastric cancer. Br J Surg 2005; 92: 886–9
- Janunger KG, Hafstrom L, Glimelius B. Chemotherapy in gastric cancer: A review and updated meta-analysis. Eur J Surg 2002; 168: 597–608
- Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, et al. Adjuvant therapy after curative resection for gastric cancer: Meta-analysis of randomized trials. J Clin Oncol 1993; 11: 1441–7
- Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: Revisiting a meta-analysis of randomised trials. Eur J Cancer 1999; 35: 1059–64
- Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: A meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente). Ann Oncol 2000; 11: 837–43
- Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, et al. Adjuvant chemotherapy in gastric cancer: A meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori 2002; 88: 21–7
- Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, et al. Intravenous chemotherapy for resected gastric cancer: Meta-analysis of randomized controlled trials. World J Gastroenterol 2002; 8: 1023–8
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725–30
- Smalley SR, Gunderson L, Tepper J, Martenson JA, Jr, Minsky B, Willett C, et al. Gastric surgical adjuvant radiotherapy consensus report: Rationale and treatment implementation. Int J Radiat Oncol Biol Phys 2002; 52: 283–93
- Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol 2002; 12: 187–95
- Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PWT, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): Quality of combined modality therapy and pathologic response. J Clin Oncol 2006; 24: 3953–8
- Ajani JA, Mayer RJ, Ota DM, Steele GD, Evans D, Roh M, et al. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. J Natl Cancer Inst 1993; 85: 1839–44
- Fink U, Schuhmacher C, Stein HJ, Busch R, Feussner H, Dittler HJ, et al. Preoperative chemotherapy for stage III-IV gastric carcinoma: Feasibility, response and outcome after complete resection. Br J Surg 1995; 82: 1248–52
- Rougier P, Mahjoubi M, Lasser P, Ducreux M, Oliveira J, Ychou M, et al. Neoadjuvant chemotherapy in locally advanced gastric carcinoma–a phase II trial with combined continuous intravenous 5-fluorouracil and bolus cisplatinum. Eur J Cancer 1994; 30A: 1269–75
- Kelsen D, Karpeh M, Schwartz G, Gerdes H, Lightdale C, Botet J, et al. Neoadjuvant therapy of high-risk gastric cancer: A phase II trial of preoperative FAMTX and postoperative intraperitoneal fluorouracil-cisplatin plus intravenous fluorouracil. J Clin Oncol 1996; 14: 1818–28
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20
- Ychou M, Pignon JP, Lasser P, Conroy T, Bouche O, Boige V, et al. Phase III preliminary results of preoperative fluorouracil (F) and cisplatin (P) versus surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC 94012-FFCD 9703 trial. J Clin Oncol 2006; 24: 185s(abstr 4026)
- Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15: 261–7
- Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 2002; 20: 1996–2004
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24: 2903–9
- Cunningham D, Rao S, Starling N, Iveson T, Nicolson M, Coxon F, et al. Randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric (OG) cancer: The REAL 2 trial. J Clin Oncol (Meeting Abstracts) 2006; 24: LBA4017
- Pozzo C, Barone C, Szanto J, Padi E, Peschel C, Bukki J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: Results of a randomized phase II study. Ann Oncol 2004; 15: 1773–81
- Bouche O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: A Federation Francophone de Cancerologie Digestive Group Study–FFCD 9803. J Clin Oncol 2004; 22: 4319–28
- Moehler M, Eimermacher A, Siebler J, Hohler T, Wein A, Menges M, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer 2005; 92: 2122–8
- Enzinger PC, Kulke MH, Clark JW, Ryan DP, Kim H, Earle CC, et al. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci 2005; 50: 2218–23
- Kohne CH, Catane R, Klein B, Ducreux M, Thuss-Patience P, Niederle N, et al. Irinotecan is active in chemonaive patients with metastatic gastric cancer: A phase II multicentric trial. Br J Cancer 2003; 89: 997–1001
- Dank M, Zaluski J, Barone C, Valvere V, Peschel C, Wenczl M. Randomized phase 3 trial of irinotecan (CPT-11) + 5FU/folininc acid (FA) vs CDP + 5FU in 1st-line advanced gastric cancer patients. J Clin Oncol 2005; 23: 308s(Abstr: 4003)
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol 2006; 24: 4991–7
- Roth AD, Maibach R, Falk S, Stupp R, Saletti P, Käberle M, et al. Docetaxel-cisplatin-5FU (TCF) versus docetaxel-cispaltin (TC) versus epirubicin-cisplatin-FU (ECF) as systemic treatment for advanced gastric carcinoma (AGC): A randomized phase II trial of the Swiss Group for Clinical Cancer Research (SAKK). Annual Meeting Proceedings ASCO 2004; 23: 317(Abstr: 4020)
- Kang Y, Kang WK, Shin DB, Chen J, Xiong J, Wang J. Randomized phase III trial of capecitabine/cisplatin (XP) vs. continuous infusion of 5-FU/cisplatin (FP) as first-line therapy in patients (pts) with advanced fgastric cancer (AGC): Efficacy and safety results. J Clin Oncol 2006; 24: 934s(Abstr:LBA4015)
- Baek JH, Kim JG, Jeon SB, Chae YS, Kim DH, Sohn SK, et al. Phase II study of capecitabine and irinotecan combination chemotherapy in patients with advanced gastric cancer. Br J Cancer 2006; 94: 1407–11
- Fuchs C, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M. A randomized trial of first-line irinotecan/fluoropyrimidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C). J Clin Oncol 2006; 24: 147s(Abstr: 3506)
- Ajani JA, Faust J, Ikeda K, Yao JC, Anbe H, Carr KL, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005; 23: 6957–65
- Ajani JA, Lee F-C, Singh DA, Haller DG, Lenz H-J, Benson AB, III, et al. Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2006; 24: 663–7
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 1995; 19: 183–232
- Doi T, Koizumi W, Siena S, Cascinu S, Ohtsu A, Michael M, et al. Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer. J Clin Oncol (Meeting Abstracts) 2003; 22
- Enzinger, PC, Fidias, P, Meyerhardt, J, Stuart, K, Fuchs, C, Huberman, M, et al. Phase II study of bevacizumab and docetaxel in metastatic esophageal and gastric cancer. Proc Gastrointestinal Am SOc Clin Oncol Symposium. 2006.
- Bramhall SR, Hallissey MT, Whiting J, Scholefield J, Tierney G, Stuart RC, et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: A randomised trial. Br J Cancer 2002; 86: 1864–70
- Shah MA, Ilson D, Kelsen DP. Thromboembolic events in gastric cancer: High incidence in patients receiving irinotecan- and bevacizumab-based therapy 10.1200/JCO.2005.81.908. J Clin Oncol ;23 2005; 23: 2574–6
- Butler, E, Shukla, V, Teh, B, Lu, H, Paulino, A. Novel strategies for improving delineation in pancreas and stomach cancer Using evidence based standardized treatment maps. Int J Radiat Oncol Biol Phys 2006;66:S309. Abstr:2179.
- Hong, T, Crowley, E, Tsai, H, Blake, M, Napolitano, B, Kachnic, L. A 3-Dimensional CT-based atlas for radiation planning in gastric cancer: Target delineation and implications for treatment planning. Int J Radiat Oncol Biol Phys 2006;66:S294. Abstr:2150.
