Abstract
To examine whether transcatheter embolization of bone metastases is an effective palliative option for patients with renal cell carcinoma (RCCa). A retrospective review of 21 patients presenting for palliative embolization of painful RCCa skeletal metastases was performed. Details regarding anatomic sites, procedural details, and embolization materials were collected. The clinical response of the patient was assessed from clinic visits and analgesic use. Thirty separate embolization procedures were used to treat 39 metastatic lesions (18 pelvic, 8 lower extremity, 3 upper extremity, 5 rib/chest wall, and 5 vertebral lesions). Five patients underwent more than one embolization. Polyvinyl alcohol was used in all 30 embolization procedures. Additional embolic materials were used in 16 of 30 procedures. A clinical response was achieved at 36 treated sites; the mean duration of the response was 5.5 months. Selective embolization of bony renal cell carcinoma metastases can provide effective palliation in a patient population which has limited therapeutic options.
There are 36 000 new cases of renal cell carcinoma (RCCa) diagnosed annually in the USA, and the incidence is increasing Citation[1], Citation[2]. Twenty to 30% of patients have metastatic disease at presentation Citation[3] and up to 40% of patients will develop osseous metastases Citation[4], Citation[5].
Osseous metastases are a major cause of morbidity, resulting in pain, decreased mobility and pathologic fracture. Palliative treatment options for osseous metastases are limited. Despite the availability of immunotherapy and the newer anti-angiogenic agents for advanced kidney cancer, most patients will either not respond, or if they do initially respond, ultimately will suffer from disease progression during treatment Citation[6], Citation[7]. Local treatment, such as radiation therapy, can result in palliation, but is limited by cumulative regional dose and may require more than one treatment session.
The technique of transcatheter embolization has been applied pre-operatively to both the primary tumor before nephrectomy Citation[8], Citation[9] and metastatic bony lesions Citation[10–13] to limit intra-operative blood loss. Selective transcatheter embolization of RCCa osseous metastases offers a reasonable and rational therapeutic approach for pain relief in this patient population. RCCa is typically a hypervascular tumor, making it easy to identify the tumor blush, and therefore providing a good indicator of progress during the procedure. In addition, this therapy has the potential to be repeated with minimal associated risk or side effects when performed by experienced interventional radiologists.
Methods
The study design was approved by the University's Institutional Review Board. As this was a retrospective review, there were no predefined eligibility criteria. The radiological and medical records of 21 patients with documented metastatic renal cell carcinoma, who presented for palliative embolization of skeletal pain secondary to metastases between 1997 and 2003, were reviewed. These patients were referred from the clinical practice of one author (BR), in whose practice 60% of patients have renal cell carcinoma. Basic demographic information was recorded. Procedural data collected included the sites of metastases treated, details regarding catheter systems, and the embolic material(s) used. Post-embolization clinic visit notes and inpatient admissions were carefully reviewed to determine symptoms and utilization of prescribed pain medications. Response was determined by recorded analgesic/narcotic use. A clinical response was defined as a 50% or greater decrease in narcotic use, and no response (NR) as less than a 50% decrease. The duration of relief was considered to end at the time of the last documented follow-up, hospice enrollment, or death. Specific survival data was not collected.
All procedures were performed from a common femoral artery approach. A diagnostic arteriogram defined the arterial anatomy of the region. Standard (4 or 5 French) angiographic catheters were used alone or in conjunction with a coaxially placed 3 French microcatheter to selectively catheterize target arteries supplying metastases. Embolization of the target vessel(s) was undertaken using particulate material, coils, or absolute alcohol either alone or in combination. Embolization was considered complete when there was stasis of intravascular contrast material and either a) a complete elimination of the tumor's hypervascular staining, or b) an 80% or greater elimination of the tumor blush compared to the initial diagnostic angiogram (definition adapted from reference Citation[10]). The later standard was used when a portion of the lesion's blood supply originated from small, less than 1 mm branches, which could not be selectively catheterized.
Metastatic lesions that underwent a repeat embolization procedure were considered for purposes of this study as a new procedure. The clinical result was then evaluated in the post procedure period in the same manner as outlined above (e.g., response vs. NR).
Admission to the hospital following our procedure was typically not for issues related to the procedure itself, but rather, to address pain control issues that had prompted their referral for embolization. After discharge, follow-up visits were scheduled per the attending oncologist, and the subjective reports of pain as well as analgesic use were charted.
Statistics
Fisher's exact test was used to evaluate the significance of PVA particle size and clinical response to the embolization procedure. The choice of this statistical measure was guided by the relatively small numbers of patients in a particular group and by the nature of the study.
Results
Twenty-one patients (14 M, 7 F; ages 38–85, mean age 56 years) underwent 30 separate embolization procedures to treat 39 metastatic lesions. Five patients underwent more than one procedure: three patients underwent two embolizations; one patient underwent three embolizations; and one patient underwent five separate embolization procedures over a ten-month period. The total number of sites embolized does not equal the number of procedures because patients may have had more than one site treated at a single encounter.
Eight patients underwent prior, non-surgical therapies. Five had regional radiation therapy; the low number of referrals was based on the personal clinical experience of the referring oncologist. Four patients underwent systemic immunotherapy, and one patient had received both therapies prior to embolization.
The anatomic distribution of the 39 metastases treated is shown in .
Table I. Anatomic distribution of metastases and clinical response to embolization
Four or 5 French catheters were used in all procedures. Microcatheters (Tracker-18/Tracker-325: Target Therapeutics, Fremont, CA; Renegade, Boston Scientific, Watertown, MA, USA) were used in 26 of 30 cases.
Polyvinyl alcohol particles (Contour or Contour SE: Boston Scientific, Watertown, MA, USA) were used in all 30 procedures. In addition, adjunct embolization agents were used in 16/30 procedures (53%) and included: stainless steel or platinum coils, n = 11; gelfoam, n = 7; absolute alcohol, n = 4; lipiodol, n = 1 ().
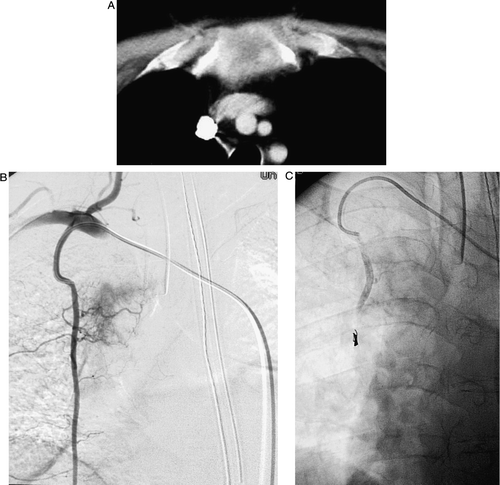
Figure 1. Forty eight-year-old M patient who presented with constant sternal and chest pain, aggravated with movement. He required continuous intravenous narcotics and was developing respiratory depression and somnolence. A) Detail from a contrast-enhanced CT scan of the chest showing the hypervascular metastatic lesion in the manubrium. B) Angiogram performed via the internal mammary arteries (IMA). The metastasis received blood supply from both the right and left IMA. C) Post-embolization angiogram of the right IMA following embolization with 45–150 uM PVA. Microcoils were deployed distally in the IMA to redirect PVA particle flow into the small feeding vessels. The left IMA injection showed a similar elimination of the tumor stain. On the morning following the procedure, the patient had his intravenous narcotics discontinued, was awake and alert, and was able to move without further pain.
Clinical aspects
A clinical response was achieved at 36 sites and there was no response (NR) in three lesions. The time to maximal response varied, but in all responding patients it occurred within 2 weeks post-embolization. The mean duration of site-specific response was 5.5 months (median, 5 months; range, 0.5–19 months) (). Those lesions which did not respond to embolization were in varied anatomical distributions (chest wall, pelvis, and lower extremity) and not clustered in any single region ().
Figure 2. This example of a pelvic metastasis (left inferior pubic ramus) was treated with 250–355 micron PVA only. The patient experienced complete resolution of his pain for 9 months. A) Pre-procedure pelvic radiograph with destruction of the left inferior pubic ramus. B) Selective left internal iliac artery angiogram demonstrating the hypervascular renal cell carcinoma metastasis. C) Post-embolization angiogram showing elimination of the hypervascularity in the region of the inferior pubic ramus. D) Follow-up post-embolization pelvic radiograph showing sclerosis of the treated metastasis. This finding is unusual, but has been reported. (19).
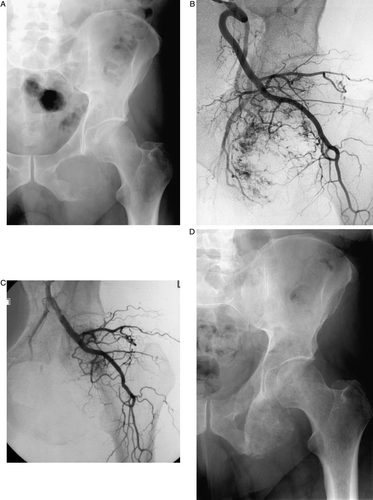
Table II. Relationship between size of PVA particles and clinical response to the embolization procedure
One patient (57 F) underwent repeat embolization of two pelvic lesions (right and left ischium) with a 7.5-month treatment interval. She achieved a response lasting 7 and 5 months respectively from the first and second embolization procedures.
summarizes PVA particle size as related to the subsequent clinical response. There was no statistically significant trend between PVA particle size and a clinical response (p-values ranged from 0.17 to 0.61 using a Fisher's exact test). Contributing to this was a low number of procedures yielding no clinical response. No conclusions can be drawn from the use of adjunct embolic agents and an improved clinical outcome. Of the NR lesions, two were embolized with PVA alone and one was treated with a combination of multiple PVA particle sizes and coils.
Complications
During the immediate post-procedure observation period, no patient exhibited clinical signs of post-embolization syndrome (fever, chills, nausea/vomiting, or increased pain at the site of embolization). There were three procedural complications. Two were classified as major by the Society of Interventional Radiology Quality Improvement Guidelines Citation[14]. A 57-year-old male patient experienced lower extremity paralysis after embolization of the L-1 vertebral body. A 58-year-old female patient experienced acute hypoxia secondary to pulmonary embolus during embolization of the T-1 vertebral body. Finally, a 47-year-old female patient suffered transient sciatic paralysis after embolization of a right ilium metastasis. This symptom resolved during her hospitalization, required no change in the type or level of care she was receiving, and was therefore classified as a minor periprocedural complication.
Discussion
Transcatheter embolization treatment of painful RCCa osseous metastases has been reported in case reports and small case series beginning in the 1970's and early 1980's. These report subjective improvement or relief of symptoms ranging from one to 9 months duration Citation[16–19]. The majority of these patients were embolized with a combination of gelfoam and metallic coils Citation[16], Citation[17], Citation[19]. Transcatheter embolization techniques have also successfully been applied to bony metastases from other hypervascular neoplasms such as thyroid Citation[20] and hepatoma Citation[21].
Microcatheters, catheter diameters in the range of 3 French, and their use with coaxial catheter techniques were first utilized in neuroangiography in the 1970's and early 1980's Citation[22]. With time, subselective cathteterization became the goal of most visceral interventions as well. Application of modern subselective catheterization and embolization techniques to palliation of pain from bony metastatic disease, while a part of current practice, has not been re-visited to evaluate its effectiveness. A series by Barton discussed embolization of bony metastases in 51 patients Citation[10]. This series combined different tumor histologies, different therapeutic intentions, and a variety of embolic materials. The heterogenous population makes it difficult to analyze the subset of patients undergoing the procedure strictly for palliation. Of these 11 patients, it was reported that seven achieved complete pain relief and the remaining four had diminished pain. The relief lasted between 2–8 months. Unfortunately, the histology of the metastases treated for palliation was not reported.
As case reports and small case series have been published over the last thirty years, some principles of embolization as applied to RCCa have been defined, namely safety, clinical benefit, and repeatability Citation[23]. No large series has investigated the clinical outcomes or described current technical aspects.
The result of an embolization procedure is partly dependent on the specific characteristics of the embolic agent. The composition of the agent, interaction between the agent and vessel wall, size, and physical nature of the embolic agent all help to determine the level of vascular occlusion. The most commonly used particle size in our study was 355–500 micron diameter; this is considered a mid-range particle size, achieving occlusion in the distal segments of the target artery. The low number of NR lesions in this study (3 of 39 total lesions) suggests that strict adherence to general principles of transcatheter embolization, such as targeted distal delivery and allowing for redistribution of particles, is more important than a specific combination of agents for a given lesion. By interrupting the blood supply to the hypervascular RCCa metastasis, tumor growth is reduced and subsequent distention or destruction of the richly innervated periosteum is slowed or stopped altogether, as suggested by Chuang Citation[16]. Additional benefits may result from decreased blood flow and the reduction of edema, which may cause direct pressure effects on adjacent structures and nerves in the surrounding tissues.
Regarding the complications, there were two major adverse outcomes and one minor adverse outcome as described above. Both major complications occurred in patients undergoing embolization of vertebral body metastases. While transcatheter embolization can be performed on these lesions, these patients should be considered at high risk for potential complications, and informed consent should include a discussion of both direct neurologic and non-neurologic complications. We experienced no incidence of post-embolization skin breakdown, as had been reported earlier by Varma Citation[19]. Post-embolization syndrome was not observed. The occurrence of these symptoms (pain, fever, nausea, vomiting), while well described with solid organ embolizations, is variably reported with bone embolizations, ranging from 18 to 86% Citation[9], Citation[10], Citation[19].
Patients with painful osseous metastases have limited therapeutic options. Preliminary evaluations have suggested that bisphosphonates may reduce skeletal related events in patients with bone metastases from kidney cancer Citation[24]. Radiation therapy has been a mainstay of therapy. To reduce the need for patients to be treated on successive visits, single-fraction radiotherapy has been evaluated and shown to be effective in treating bony metastases from lung and breast carcinoma Citation[25]. In this study, a single treatment with 8 Gy was as efficacious as a total dose of 30 Gy over 10 fractions, with an overall clinical response rate of 66%. Historically, RCCa has been thought of as a radioresistant tumor, based on in vitro studies comparing the radiosensitivity of various tumor cell lines Citation[26]. A more recent study reports objective palliative responses to radiation therapy of RCCa bony metastases using the modified McGill-Melzack scale with a 3 month median duration of response in 83% of patients evaluated Citation[27]. Stereotactic radiotherapy may hold promise for both symptom and local tumor control in RCCa Citation[28], but no experience with treatment of osseous lesions is available
The clinical response rate of 92% and mean duration for symptom improvement of 5.5 months observed in our patients undergoing transcatheter embolization compares very favorably with the reported response rate from RTOG 9714 of 66% Citation[25]. Our report is a retrospective review and did not utilize a predefined quantitative assessment tool. However, subjects were followed by a single clinical team, and chart review of documented narcotic prescriptions requiring a 50% or greater decrease in narcotic use was utilized. A prospective comparison of the two therapeutic approaches would be needed to determine if one pathway is superior to the other in terms of degree of response, durability of the response, and impact on quality of life.
In summary, selective embolization of osseous RCCa metastases is an effective technique that can provide palliative relief for a patient population with limited therapeutic options. The therapy can be repeated with equal success.
Conflict of interest statement
None declared by any of the authors.
References
- Chow WH, Devesa SS, Warren JL. Rising incidence of renal cell cancer in the United States. JAMA 1999; 281: 1628–31
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics. CA-A Cancer J Clin 2004; 54: 8–29
- Vogelzang NJ, Stadler WM. Kidney Cancer. Lancet 1998; 352: 1691–6
- Saitoh H. Distant metastasis of renal adenocacinoma. Cancer 1981; 48: 1487–91
- Swanson DA, Orovan WL, Johnson DE, Giacco G. Osseous metastases secondary to renal cell carcinoma. Urology 1981; 18: 556–61
- Vogelzang NJ. Treatment options in metastatic renal carcinoma: An embarrassment of riches. J Clin Oncol 2006; 24: 1–3
- Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2. Ann Surg 1998; 228: 307–19
- Almgard LE, Fernstrom I, Haverling M, Ljungqvist A. Treatment of renal adenocarcinoma by embolic occlusion of the renal circulation. Br J Urol 1973; 45: 474–9
- Christensen K, Dyreborg U, Andersen JF, Nissem HM. The value of transvascular embolization in the treatment of renal carcinoma. J Urol 1985; 133: 191–3
- Barton PP, Waneck RE, Karnel FJ, Ritschl P, Kramer J, Lechner GL. Embolization of bone metastases. JVIR 1996; 7: 81–8
- Sun S, Lang EV. Bone metastases from renal cell carcinoma: Preoperative embolization. JVIR 1998; 9: 263–9
- Bowers TA, Murray JA, Charnsangavej C, Soo CS, Chuang VP, Wallace S. Bone metastases from renal carcinoma: The preoperative use of transcatheter arterial occlusion. J Bone Joint Surg 1982; 64: 749–54
- Chatziioannou AN, Johnson ME, Pneumaticos SG, Lawrence DD, Carrasco CH. Preoperative embolization of bone metastases from renal cell carcinoma. Euro Radiol 2000; 10: 593–6
- Drooz AT, Lewis CA, Allen TE, Citron SJ, Cole PE, Freeman NJ, et al. Quality improvement guidelines for percutaneous transcatheter embolization. J Vasc Interv Radiol 2003; 14: S237–S242
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003; 21: 3127–32
- Chuang VP, Wallace S, Swanson D, Zoroza J, Handel SF, Schwarten DA, Murray J. Arterial occlusion in the management of pain from metastatic renal carcinoma. Radiology 1979; 133: 611–4
- Wallace S, Granmayeh M, DeSantos LA, Murray JA, Romsdahl MM, Bracken RB, et al. Arterial occlusion of pelvic bone tumors. Cancer 1979; 43: 322–8
- Weber J. Palliative embolization in bone metastasis of hypernephroma using oily contrast-labeled gel. Ann Radiol 1982; 25: 460–2
- Varma J, Huben RP, Wajsman Z, Pontes JE. Therapeutic embolization of pelvic metastases of renal cell carcinoma. J Urol 1984; 131: 647–9
- Eustatia-Rutten CF, Romijn JA, Guijt MJ, Vielvoye GJ, van den Berg R, Corssmit EPM, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrin Metab 2003; 88: 3184–9
- Uemura A, Fujimoto H, Yasuda S, Osaka I, Goto N, Shinozaki M, et al. Transcatheter arterial embolization for bone metastases from hepatocellular carcinoma. Euro Radiol 2001; 11: 1457–62
- Berenstein A, Kricheff II. Catheter and material selection for transarterial embolization: Technical considerations. Radiology 1979; 132: 619–30
- Yilmaz S, Sindel T, Luleci E. Repeated palliative embolization of renal cell carcinoma metastases. Clin Radiol 2002; 57: 319–20
- Lipton A, Colombo-Berra A, Bukowski RM, Rosen L, Zheng M, Urbanowitz G. Skeletal complications in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin Can Res 2004; 10(Suppl): s6397–s6403
- Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach III M, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005; 97: 798–804
- Onufrey V, Mohiuddin M. Radiation therapy in the treatment of metastatic renal cell carcinoma. Int J Radiat Oncol Biol Phys 1985; 11: 2007–9
- Lee J, Hodgson D, Chow E, Bezjak A, Catton P, Tsuji D, et al. A phase II trial of palliative radiotherapy for metastatic renal cell carcinoma. Cancer 2005; 104: 1894–900
- Svedman C, Sandstrom P, Pisa P, Blomgren H, Lax I, Kalkner K-M, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006; 45: 870–5
