Abstract
While the early diagnosis of cancer has been fully respected, it is still however often difficult for clinicians to confirm malignant pleural effusions (PE), which essentially indicate the end-stage cancer. It has now been demonstrated that vascular endothelial growth factor (VEGF) is a pivotal angiogenesis factor and associated with tumor growth and metastasis. The aim of this study was then to assess the diagnostic performance of VEGF in malignant PE. In this controlled and blinded prospective study, 113 consecutive patients with PE were recruited. For each eligible case, the VEGF levels of pleural fluid (PF) and serum were examined simultaneously using enzyme immunoassay. The reference standard for malignant PE was clinical evaluation and PF cytology with pleural biopsy, other examination and follow-up added as needed. According to the final diagnoses, 81 qualified cases were grouped as malignant (n=32) and benign (n=49) PE. For PF VEGF level, the mean in malignant group was higher than that in benign group (1358±1493 pg/mL vs. 422±317 pg/mL, p=0.001). As did for serum VEGF level (650±533 pg/mL vs. 137±189 pg/mL, p<0.001). Using receiver operating characteristic analysis, the determined diagnostic cut-off points of VEGF levels of PF and serum for malignant PE were 959.25 pg/mL and 212.36 pg/mL, with sensitivities of 47%, 69% and specificities of 96%, 88%, respectively. For cascade connection and parallel operation of PF VEGF and serum VEGF, the sensitivities were 34%, 81% at specificities of 98%, 86%, respectively. These findings suggest that VEGF could be used in diagnosing malignant PE as a useful adjunct of conventional algorithm. Different VEGF test strategies, including test on PF, serum and both, may be selected according to practical needs.
Approximately 50% of patients with metastatic cancer develop pleural effusions (PE) clinically Citation[1]. The presence of malignant PE usually indicates the severity of illness and a short survival time Citation[2]. Even for the patients with such end-stage cancer, however, the confirmation of malignant PE is often a knotty problem.
Currently, the simplest definitive method for diagnosing malignant PE is cytological examination of pleural fluid (PF). The specificity of PF cytology is commonly high, but the sensitivity, reported ranging from 30 to 90%, is insufficient for practical needs Citation[3], Citation[4]. When cytology is negative, 7 to 12% of malignant PE may be confirmed by closed pleural biopsy Citation[3]. Medical thoracoscopy is of further diagnostic value in cases of undiagnosed exudative PE with a high clinical suspicion for malignancy. However, <10% of cases remain undiagnosed after thoracoscopy, whereas >20% are still undiagnosed with cytology and closed needle biopsy Citation[3]. In practice, even for ∼15% of patients with exudative PE, no diagnosis is ever established despite invasive procedures such as thoracoscopy or open pleural biopsy Citation[5]. Confronted with such a problem, we are interested in new diagnostic approaches in medical field, such as the test of possible tumor markers. The reliable and easily used biomarkers for most types of cancer are thus eagerly sought.
It has now been demonstrated that vascular endothelial growth factor (VEGF) is a pivotal angiogenesis factor and associated with tumor growth and metastasis Citation[6–10]. While secreted by nearly all cell types, VEGF is overexpressed by most malignant tumors and at lower levels in many normal adult tissues Citation[1], Citation[11]. The VEGF level of human malignant PE correlates with the volume of PE Citation[12], Citation[13]. Then, could VEGF be used efficiently in the diagnosis of malignant PE? There have been several different and inconsistent answers implicated in some previous reports Citation[1], Citation[7], Citation[14–17]. We hypothesized that VEGF is in all probability helpful in assisting the diagnosis of malignant PE. With the aim to estimate the diagnostic accuracy of VEGF in malignant PE, we performed this controlled and blinded prospective study.
Material and methods
Patients and specimens
Approved by the local Ethics Committee for Research, this prospective study was set out on May 2003. From then to April 2004, 172 patients with PE diagnosed in the outpatient department and without previous treatments were consecutively admitted to our university affiliated, tertiary teaching hospital. Upon informed consent, 113 were recruited immediately when hospitalized. For each recruited patient, during the course of routine diagnostic procedures and upon the patient's informed consent, 10 mL PF and 2 mL serum would be saved simultaneously for VEGF test while the first thoracentesis was performed if possible. Given the patient's informed consent or qualified specimens unavailable, this case would then be excluded. As would happen if the clinically definite etiological diagnosis of PE or the laboratory conclusive result of index test was absent finally. The clinical diagnostic procedures for recruited patients included routine biochemical analysis and cytological examination of PF. Pleural biopsy, bronchoscopy, lung biopsy and other examination were utilized when necessary. A team, though not always the same team in each case, consisting of three to four well trained and experienced chest physicians including at least one chief physician, made the final diagnosis for each recruited patient. The reference standard for malignant PE was clinical evaluation and PF cytology, combined with pleural biopsy, other examination and follow-up as needed.
The laboratory VEGF test was completely independent of clinical diagnosis and treatment. The readers of the index tests and reference standard were blind to the results of the other test. Only after completion of all the experimental tests of VEGF and clinical data collection, we analyzed the results of VEGF test in accordance with those of reference standard.
Methods of VEGF test on PF and on serum
When obtained, each fresh specimen of PF or serum underwent pretreatment instantly. After centrifugation at 3000 rpm, 4°C for 5 minutes, the supernatant of PF or serum was transferred to new tubes and reserved at −70°C, ready for concentrated test. For the VEGF test on PF and on serum, we used the commercial enzyme immunoassay (EIA) kits for the quantitative determination of VEGF in tissue homogenate and human serum, respectively (LIFEKEY BioMeditech Corp., Ames, IA, USA). Strictly following the directions of the above kits, three well-trained and skilled analysts familiar with techniques of cellular and molecular immunology performed this study. With MATLAB software (The MathWorks, Inc., Natick, MA, USA), the relation between the optical density (OD) readings at 450 nm in standard samples and their corresponding VEGF levels was primarily analyzed in each of VEGF tests on PF and serum. The unknown concentrations of VEGF in those examined samples were then calculated through OD readings accordingly.
Statistical analysis
For each VEGF test strategy, the results in malignant and benign groups were firstly described and compared. If there was a statistical difference, the sensitivity and specificity of this test for malignant PE, including rates of sample and 95% confidence intervals, were reached by counting. For measurement data, the diagnostic cut-off point was primarily calculated via receiver operating characteristic (ROC) curve. Statistical evaluation was performed by computer analysis with SPSS software (SPSS Inc., Chicago, IL, USA). To estimate the differences between different groups, χ2 test was selected for numeration data while t-test for measurement data. The statistical significance was set at p < 0.05 (2-sided or 2-tailed).
Results
Categorization of qualified participants according to reference standard
Of the 172 patients satisfying the criteria for inclusion, 91 were finally excluded in conformity to the exclusion criteria. The number of eligible patients that did or did not undergo the index tests and/or the reference standard is reported as a flow diagram (). According to the final diagnoses, the 81 qualified participants were grouped under two heads: malignant (n = 32) and benign (n = 49) PE. The key clinical and demographic characteristics of the study population in both groups are shown and compared ().
Figure 1. A flow diagram for participants. The number of participants satisfying the criteria for inclusion that did or did not undergo the index tests and/or the reference standard is reported here. The reasons why participants failed to receive either test are also described.
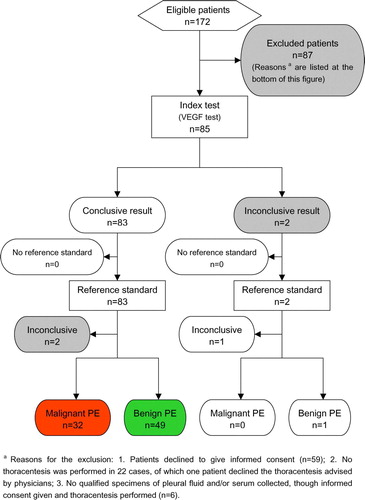
Table I. Key clinical and demographic characteristics of the study population [Means (SDs) except as noted].
In malignant PE group, the underlying diseases were: primary bronchogenic carcinomas (n = 18), adenocarcinomas of unknown origins (n = 7), cancerous cells of indefinite origins discovered in PF, but their types not identified clearly (n = 4), breast carcinoma (n = 1), non-Hodgkin's lymphoma (n = 1), squamous carcinoma of unknown origin (n = 1); In benign PE group, the etiological diagnoses were: tuberculous pleurisy (n = 38), rheumatoid arthritis (n = 3), hydropneumothorax (n = 2), pneumonia (n = 1), systemic lupus erythematosus (SLE) (n = 1), cardiac insufficiency (n = 2), hypoalbuminemia caused by hepatic cirrhosis (n = 1), nephrotic syndrome (n = 1).
Results of VEGF test on PF, serum and both
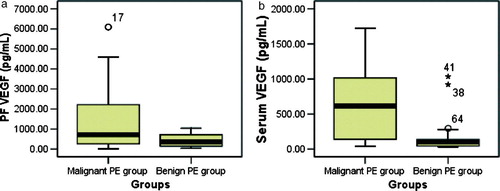
The standard curves are shown (A). The results of VEGF test on PF and serum in both malignant and benign PE group are also described and compared (). For PF VEGF level, the mean in malignant PE group was higher than that in benign PE group (1358±1493 pg/mL vs. 422±317 pg/mL, p = 0.001). Similarly for serum VEGF level (650±533 pg/mL vs. 137±189 pg/mL, p<0.001). However, there was no statistical difference in the ratio of PF VEGF to serum VEGF between groups (6.03±14.50 vs. 6.00±6.06, p>0.05).
Figure 2. Standard curves and ROC curves in VEGF tests. A, (a) and (b) indicate the standard curves in VEGF tests on PF and serum, respectively; B, (c) and (d) show the ROC curves in VEGF tests on PF and serum, with the areas under the curves 0.686 (p = 0.005) and 0.834 (p < 0.001), respectively.
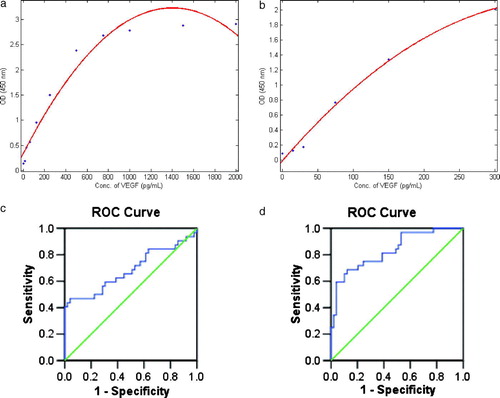
Figure 3. Box-whisker plots representing the results of VEGF test on PF (a) and serum (b) in both groups. For PF VEGF level, the mean in malignant group was higher than that in benign group (1358±1493 pg/mL vs. 422±317 pg/mL, p = 0.001). As did for serum VEGF level (650±533 pg/mL vs. 137±189 pg/mL, p < 0.001).
Using ROC analysis (B), the optimal cut-off points of VEGF test on PF and serum in diagnosing malignant PE were calculated. The diagnostic cut-off point of PF VEGF level was 959.25 pg/mL, with a sensitivity of 47% and a specificity of 96%. That is, a PF VEGF level was judged as positive and indicated malignant PE if it was greater than or equal to this point. For serum VEGF level, the diagnostic cut-off point was 212.36 pg/mL (positive and indicative of malignancy if greater than or equal to this), with a sensitivity of 69% and a specificity of 88%.
With the above cut-off points, the number of qualified participants in both malignant and benign PE group that had positive or negative test results is described (). Provided that we determined a case as malignant when both the PF VEGF level and the serum VEGF level indicated malignancy, whereas a benign case when any of the above two was negative, the sensitivity and specificity of this combination test strategy (cascade connection of PF VEGF test and serum VEGF test) were 34% and 98%, respectively; Given that we determined a case as malignant when any of the PF VEGF level and the serum VEGF level indicated malignancy, whereas a benign case only when both negative, the sensitivity and specificity of this combination test strategy (parallel operation of PF VEGF test and serum VEGF test) were 81% and 86%, respectively ().
Table II. Results of VEGF test with the diagnostic cut-off points.
Table III. Sensitivities and specificities of different VEGF test strategies [Rates of sample (95% confidence intervals)].
In addition, no adverse events emerged from performing VEGF test or the reference standard.
Discussion
The results of this study demonstrate that, for PF VEGF, the level in malignant PE is generally higher than that in benign PE. Similarly for serum VEGF. Why? During the course of tumor growth and metastasis, it is essential that angiogenesis develops. VEGF is well established as one key regulator of this process. Through the VEGF pathway, a network of signaling processes promoting growth, migration and survival from pre-existing vasculature of endothelial cells may be triggered. In addition, VEGF mediates vessel permeability, and is associated with malignant effusions Citation[10]. The production of malignant PE requires tumor cells to invade the pleura and express high levels of VEGF Citation[12]. Not only pathological but also developmental and physiological neovascularization does VEGF mediate Citation[6], Citation[12]. Hence, VEGF could be detected in both malignant and benign conditions. Since VEGF is overexpressed by most malignant tumors, the VEGF level in malignant group may thus be heightened compared with that in benign group. Based upon the significant elevation of VEGF level of PF or serum in malignant group, we speculate that VEGF might serve as a biomarker for most malignant conditions, and not limited to malignant PE. As for the accumulation of VEGF in PF, not only mesothelial cells, infiltrating inflammatory cells and in malignant PE, cancer cells but also adjacent epithelial cells, type II alveolar cells, alveolar macrophages, infiltrating neutrophils and eosinophils of the lung may contribute to it Citation[17]. With respect to no statistical difference in the ratio of PF VEGF to serum VEGF between groups that might be resulted from the parallel elevation of VEGF levels of PF and serum in malignancy.
In the results of this study, however, there were also overlaps in the VEGF levels of PF and serum between malignant and benign groups. Some malignant cases might have lower levels of VEGF, while higher VEGF levels might be present in some benign cases. Why then? The former might be because that, we postulate, detectable VEGF is overexpressed by most malignant tumors, but probably not by all types of human cancer, especially when combined with complications that can lead to decreased level of VEGF. Previous studies suggested that, even for those malignant tumors of different types or histology with overexpression of VEGF, the VEGF levels vary Citation[7], Citation[15]. Some circumstances, such as the initial phase of acute lung injury, may result in downregulation of VEGF level, in which endothelial permeability could be limited partly Citation[18]. As for the latter, there are possibly some reasonable explanations on the basis of relevant literatures. As mentioned above, VEGF is a multifunctional cytokine with critical roles in vasculogenesis and in both pathological and physiological angiogenesis and lymphangiogenesis Citation[19]. Excluding malignancy, some other pathological conditions, such as hypoxia, pulmonary embolism, hemorrhagic effusions and empyema, could also lead to or present with heightened levels of VEGF Citation[15], Citation[16], Citation[20]. VEGF also has a central role in wound healing and inflammation Citation[11]. In tuberculous pleurisy, mycobacteria may cause VEGF release from mesothelial cells and lead to protein exudation by altering mesothelial adherens junction proteins Citation[21].
With the exception of some cases, the vast majority of malignant cases in this study had higher levels of VEGF, and benign cases had lower levels. Followed by statistical analysis, it was crucial to determine the optimal diagnostic cut-off points of VEGF level. Normally, different cut-off points lead to different sensitivities and specificities. A relatively lower point may increase the sensitivity of detection of malignant PE, but potentially at the expense of decreased specificity; vice versa. The ROC curve is a plot representing the relationship between “true positive fraction” (sensitivity) and “false positive fraction” (1-specificity) of a diagnostic test over all possible cut-off points. The very cut-off point that may have the optimal balance of maximized sensitivity and minimized “1-specificity” would be determined as ideal. In fact, among all possible cut-off points, it would have the largest Youden Index (sensitivity + specificity-1) for a diagnostic test to use the determined point.
From the results of statistical analysis, including the ROC analysis, we can preliminarily confirm the conclusion that VEGF could be utilized in the diagnosis of malignant PE. Further, in the light of the sensitivities and specificities, the clinical utility of VEGF in diagnosing malignant PE could be seen clearly, though the diagnostic accuracy is still insufficient for clinical needs. Since VEGF tests on PF and serum are both easily performed, they might be potentially applied to clinical practice for screening of malignant PE in combination with currently conventional diagnostic tools. Interestingly, the serum VEGF test prevailed over the PF VEGF test in Youden Index (0.57 vs. 0.43). The PF VEGF test, however, had a better positive likelihood ratio (11.75 vs. 5.75), while its negative likelihood ratio was inferior to that of serum VEGF test (0.55 vs. 0.35). For combination tests, there are also a tradeoff between the sensitivity and specificity, where the likelihood ratio and Youden Index may be used for overall assessment of test strategies. For cascade connection and parallel operation of PF VEGF test and serum VEGF test, the former had the most predominant positive likelihood ratio (17), whereas the latter possessed the most superior Youden Index (0.67) and negative likelihood ratio (0.22). These different test strategies may be selected according to practical purposes.
In particular, the ratio of female to male in malignant group in this study was higher than that in benign group. This might be associated with the selectiveness of samples. Compared with previous studies, the PF VEGF level in malignant group here is very close to some reported Citation[7], Citation[15]. Strictly complying with the principles of clinical epidemiology for a diagnostic test, this study has acquired the diagnostic accuracy of VEGF in malignant PE with the cut-off points obtained by ROC analysis. Moreover, with assessment of different VEGF test strategies, our findings might shed new light on the clinical value of VEGF in diagnosing malignant PE. Nevertheless, 91 out of 172 patients satisfying the inclusion criteria had to be excluded finally, which might influence the representativeness of samples of this study.
In conclusion, VEGF could be used in diagnosing malignant PE as a useful adjunct of conventional algorithm. Different VEGF test strategies, including test on PF, serum and both, may be selected according to practical needs. Further research, such as studies on clinical utility of detailed components of VEGF and its receptors in diagnosing malignant PE from both mRNA and protein level, would be warranted. Further investigation on the formation of PE, especially malignant PE, may also provide us with new insights into the diagnosis of malignant PE.
Acknowledgements
We thank many of our colleagues for their assistance in collecting the qualified specimens. We also gratefully acknowledge the patients and their relatives who ever provided us with convenience in this study, especially in the process of clinical follow-up investigation.
This study was supported in part by Sci-Tech Research Foundation for Outstanding Young Scientists (2002–12) from Anhui Science & Technology Department, China.
References
- Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JB, Jr, et al. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 1999; 5: 3364–8
- Benani CP, Pajeau TS, Bennett CL. Treating malignant pleural effusions cost consciously. Chest 1998;113(1 Suppl):S78–S85.
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000; 162: 1987–2001
- Braunschweig R, Yan P, Guilleret I, Delacretaz F, Bosman FT, Mihaescu A, et al. Detection of malignant effusions: Comparison of a telomerase assay and cytologic examination. Diagn Cytopathol 2001; 24: 174–80
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346: 1971–7
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380: 439–42
- Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax 1999; 54: 707–10
- Fragoso R, Pereira T, Wu Y, Zhu Z, Cabecadas J, Dias S. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood 2006; 107: 1608–16
- Lee TH, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 2003; 278: 5277–84
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005; 23: 1011–27
- Dvorak HF. Discovery of vascular permeability factor (VPF). Exp Cell Res 2006; 312: 522–6
- Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, et al. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol 2000; 157: 1893–903
- Gary Lee YC, Melkerneker D, Thompson PJ, Light RW, Lane KB. Transforming growth factor beta induces vascular endothelial growth factor elaboration from pleural mesothelial cells in vivo and in vitro. Am J Respir Crit Care Med 2002; 165: 88–94
- Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 1999; 85: 178–87
- Cheng D, Rodriguez RM, Perkett EA, Rogers J, Bienvenu G, Lappalainen U, et al. Vascular endothelial growth factor in pleural fluid. Chest 1999; 116: 760–5
- Ishimoto O, Saijo Y, Narumi K, Kimura Y, Ebina M, Matsubara N, et al. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology 2002; 63: 70–5
- Sack U, Hoffmann M, Zhao XJ, Chan KS, Hui DS, Gosse H, et al. Vascular endothelial growth factor in pleural effusions of different origin. Eur Respir J 2005; 25: 600–4
- Maitre B, Boussat S, Jean D, Gouge M, Brochard L, Housset B, et al. Vascular endothelial growth factor synthesis in the acute phase of experimental and clinical lung injury. Eur Respir J 2001; 18: 100–6
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20: 4368–80
- Mohammed KA, Nasreen N, Hardwick J, Logie CS, Patterson CE, Antony VB. Bacterial induction of pleural mesothelial monolayer barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2001; 281: L119–25
- Mohammed KA, Nasreen N, Hardwick J, Van Horn RD, Sanders KL, Antony VB. Mycobacteria induces pleural mesothelial permeability by down-regulating beta-catenin expression. Lung 2003; 181: 57–66
