To the Editor
Re-irradiation of local tumor recurrence after previous radical or adjuvant radiotherapy is often problematic because of the risk of high grade toxicity in normal tissues and inherent or acquired radioresistance of tumors. Using standard fractionation, re-treatment total doses remain low and responses are limited Citation[1–7].
Several tumor cell lines, many of them considered radioresistant, have shown excessive low-dose hyper-radiosensitivity (LD HRS) at fraction doses ≤0.5 Gy, followed by increased radioresistance at doses 0.5–1 Gy. Beyond 1 Gy, there is the usual downward bending survival curve with increasing dose Citation[8–10]. Recovery of LD HRS after 3–4 hour interval allows delivery of successive small doses 3–4 times daily Citation[11], Citation[12].
DNA double-strand breaks (DSBs) are considered as the prime lesions for potential cell death after radiation exposure Citation[8]. The integrity of DNA is tightly monitored at several checkpoints in the G1, S and G2 phases, and damages are effectively repaired by a number of mechanisms Citation[13], Citation[14], which to a large extent are regulated by the ataxia teleangiectasia mutated (ATM) protein Citation[15], Citation[16]. It seems that using low doses of ionizing radiation, the activation of ATM and the function of its downstream target histone H2AX Citation[17], Citation[18] is limited allowing the tumor cells harboring DNA DSBs to pass the second G2/M checkpoint Citation[14], Citation[19] and proceed into mitosis without being repaired Citation[14], Citation[20–22]. However, recent data suggests that LD HRS is not a result from the failure of cells to recognize DNA DSBs due to the non-functional ATM Citation[23]. Several other explanations, including local immune responses or tumor cell hypoxia, have also been proposed as possible mechanisms of LD HRS Citation[24].
Based on the rationale of a high proportion of radiation-damaged G2 phase tumor cells entering mitosis, together with a low proportion of cells in the vulnerable G2 phase in normal tissues, we have used low-dose ultrafractionated radiotherapy (LDUF RT) in selected patients with a symptomatic tumor recurrence in the previously irradiated area to achieve effective palliation but minimal normal tissue toxicity.
Patients, methods and treatment outcome
The characteristics and tumor details, given treatments, clinical and radiological responses, and observed toxicity of 11 adult patients are described in and . Full information of the experimental nature of the treatment and alternative treatment options was provided before patients’ agreement to receive LDUF RT, and the ethical standards of the Helsinki Declaration were followed. Clinical condition of the patients was graded according to the NCI common toxicity criteria (CTC), and tumor responses were evaluated using RECIST. Acute toxicity and late morbidity were assessed using NCI CTC and RTOG/EORTC scoring system, respectively. All patients were treated with three-dimensional conformal beam radiotherapy. Three fractions of 0.5 Gy (nine patients), 0.6 Gy (one patient), and 0.66 Gy (one patient) were given daily 4 h apart, 15 fractions per week. The total number of fractions per treatment varied from 60 to 102, the total radiation dose between 30 and 51 Gy, and the treatment time from 28 to 46 days. The interval from prior irradiation to LDUF RT varied from 1 to 18 years.
Table I. The characteristics of the patients with recurrent malignant tumors of the central nervous system, and the description of low-dose ultrafractionated radiotherapy, anti-tumor response, and toxicity.
Five patients (#1–#5) had an intracranial malignancy (). Prior to LDUF RT, all had undergone surgery at least twice due to a local recurrence, and three had received chemotherapy. LDUF RT caused no acute toxicity or late morbidity, and resulted in three complete responses up to duration of nearly 5 years (). Also, a striking and long-lasting reduction of clinical symptoms was observed in every patient.
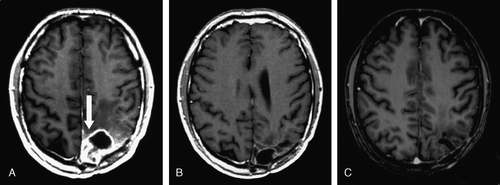
Figure 1. Imaging of the anti-tumor response of low-dose ultrafractionated radiotherapy. (A) A patient (, #1) with a rapid recurrence of grade II oligodendroglioma (arrow) 4 months after the second operation and 2 weeks before LDUF RT. A complete response is seen (B) 6 months and (C) 2 years later. T1-weighted contrast-enhanced MRIs.
Three patients (#6–#8) had rectal cancer (). All had received at least two regimens of chemotherapy, and two had been re-operated due a local recurrence. Despite multiple treatments, they suffered from local residual tumor growth causing severe pain and complicated fistulae in the gluteal and coccygeal region. Although large volumes (80% isodose volume 1265–2640 ml, including planning target volume) of pelvic region were irradiated, no toxicity related to re-irradiation was observed, and a clear relief of disabling symptoms, lasting up to one year, was achieved.
Table II. The characteristics of the patients with recurrent rectal cancer, and the description of low-dose ultrafractionated radiotherapy, anti-tumor response, and toxicity.
Regarding other type of tumors (), an elderly patient with operated renal cancer and low respiratory function (#9) received LDUF RT for a large recurrent lung metastasis (field portals 10.5×12.0 cm) resulting in partial response and significant palliation of symptoms. Also, a patient with supraclavicular lymph node metastases from breast cancer (#11) experienced relief of pain, but a patient with osteosarcoma of the sacrum (#10) did not benefit from the treatment. Again, these patients did not present any radiation-related toxicity.
Table III. The characteristics of the patients with other type of recurrent tumors treated using low-dose ultrafractionated radiotherapy.
Conclusions
LD HRS has been demonstrated clinically effective in metastatic tumor nodules of skin Citation[24]. However, this is the first publication analyzing toxicity and palliative efficacy of the LDUF RT in the treatment of recurrent tumors managed previously with surgery, chemotherapy, and conventional radical or adjuvant radiotherapy. Traditionally, palliative limited-field irradiation to a total dose of about 30 Gy is offered to these patients, if any therapy at all.
The total dose of external beam re-irradiation for primary brain tumors varies typically between 35–40 Gy with a fraction size of 1–3 Gy resulting in mean overall response rates of 40–50% Citation[1–4]. However, these treatments have caused severe acute toxicity and a variety of late complications including profound neurological injury, increased intracranial pressure, and necrosis in 10–30% of the patients during a median survival between 9 and 36 months.
The re-treatment doses for rectal cancer have ranged from 30 to 36 Gy using a fraction size of 1.8 Gy or hyperfractionation with 1.2 Gy twice a day Citation[5–7]. These studies have demonstrated marked response rates for local control and pain relief, and survival extending up to 3 years. However, numerous treatment-related adverse events like diarrhoea, skin and mucosal reaction, abscess, small bowel obstruction, fistula, coloanal stricture, and ulceration have been reported. It is probable that a certain amount of these complications are also tumor-related.
It has been shown that only G2 cells exhibit LD HRS Citation[21] and therefore tumors with a high G2 content, which are expected to have a low potential doubling time and high cell loss factor Citation[25], should show more effect of reducing the fraction size. This could explain why the most significant benefit and anti-tumor response from LDUF RT was achieved in patients with malignant glioma.
The treatment schedule of LDUF RT is demanding for the patients, and increased labor and limited accelerator capacity restricts its use in daily practice. However, based on the experience of our small series of patients, LDUF RT is a safe option for effective palliation with minimal toxicity in selected patients with locally recurrent tumors after conventionally fractionated radical or adjuvant radiotherapy.
References
- Arcicasa M, Roncadin M, Bidoli E, Dedkov A, Gigante M, Trovo MG. Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Phys 1999; 43: 789–93
- Dritschilo A, Bruckman JE, Cassady JR, Belli JA. Tolerance of brain to multiple courses of radiation therapy. I. Clinical experiences. Br J Radiol 1981; 54: 782–6
- Bauman GS, Sneed PK, Wara WM, et al. Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys 1996;36:433–41.
- Kim HK, Thornton AF, Greenberg HS, Page MA, Junck L, Sandler HM. Results of re-irradiation of primary intracranial neoplasms with three-dimensional conformal therapy. Am J Clin Oncol 1997; 20: 358–63
- Mohiuddin M, Lingareddy V, Rakinic J, Marks G. Reirradiation for rectal cancer and surgical resection after ultra high doses. Int J Radiat Oncol Biol Phys 1993; 27: 1159–63
- Lingareddy V, Ahmad NR, Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys 1997; 38: 785–90
- Mohiuddin M, Marks GM, Lingareddy V, Marks J. Curative surgical resection following reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys 1997; 39: 643–9
- Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys 2001; 49: 379–89
- Beauchesne PD, Bertrand S, Branche R, et al. Human malignant glioma cell lines are sensitive to low radiation doses. Int J Cancer 2003;105:33–40.
- Short S, Mayes C, Woodcock M, Johns H, Joiner MC. Low dose hypersensitivity in the T98G human glioblastoma cell line. Int J Radiat Biol 1999; 75: 847–55
- Mitchell CR, Joiner MC. Effect of subsequent acute-dose irradiation on cell survival in vitro following low dose-rate exposures. Int J Radiat Biol 2002; 78: 981–90
- Short SC, Kelly J, Mayes CR, Woodcock M, Joiner MC. Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int J Radiat Biol 2001; 77: 655–64
- Zhou BB, Elledge SJ. The DNA damage response: Putting checkpoints in perspective. Nature 2000; 408: 433–9
- Xu B, Kim ST, Lim DS, Kastan MB. Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol 2002; 22: 1049–59
- Shiloh Y. ATM and related protein kinases: Safeguarding genome integrity. Nat Rev Cancer 2003; 3: 155–68
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421: 499–506
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: The histone guardian of the genome. DNA Repair (Amst) 2004; 3: 959–67
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003; 5: 675–9
- Marples B, Wouters BG, Joiner MC. An association between the radiation-induced arrest of G2-phase cells and low-dose hyper-radiosensitivity: A plausible underlying mechanism?. Radiat Res 2003; 160: 38–45
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA 2003; 100: 5057–62
- Marples B, Wouters BG, Collis SJ, Chalmers AJ, Joiner MC. Low-dose hyper-radiosensitivity: A consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat Res 2004; 161: 247–55
- Enns L, Bogen KT, Wizniak J, Murtha AD, Weinfeld M. Low-dose radiation hypersensitivity is associated with p53-dependent apoptosis. Mol Cancer Res 2004; 2: 557–66
- Wykes SM, Piasentin E, Joiner MC, Wilson GD, Marples B. Low-dose hyper-radiosensitivity is not caused by a failure to recognize DNA double-strand breaks. Radiat Res 2006; 165: 516–24
- Harney J, Short SC, Shah N, Joiner M, Saunders MI. Low dose hyper-radiosensitivity in metastatic tumors. Int J Radiat Oncol Biol Phys 2004; 59: 1190–5
- Shibamoto Y, Nishimura Y, Tsutsui K, Sasai K, Takahashi M, Abe M. Comparison of accelerated hyperfractionated radiotherapy and conventional radiotherapy for supratentorial malignant glioma. Jpn J Clin Oncol 1997; 27: 31–6
