Abstract
Addition of thoracic radiation therapy (TRT) to chemotherapy (CHT) can increase overall survival in patients with small cell lung cancer limited-disease (SCLC-LD). Accelerated fractionation and early concurrent platinum-based CHT, in combination with prophylactic cranial irradiation, represent up-front treatment for this group of patients. Optimised and tailored local and systemic treatment is important. These concepts were applied when a new regional treatment programme was designed at Sahlgrenska University Hospital in 1997. The planned treatment consisted of six courses of CHT (carboplatin/etoposide)+TRT±prophylactic cranial irradiation (PCI). Standard TRT was prescribed as 1.5 Gy BID to a total of 60 Gy during 4 weeks, starting concomitantly with the second or third course of CHT. However, patients with large tumour burdens, poor general condition and/or poor lung function received 45 Gy, 1.5 Gy BID, during 3 weeks. PCI in 15 fractions to a total dose of 30 Gy was administered to all patients with complete remission (CR) and “good” partial remission (PR) at response evaluation.
Eighty consecutive patients were treated between January 1998 and December 2004. Forty-six patients were given 60 Gy and 34 patients 45 Gy. Acute toxicity occurred as esophagitis grade III (RTOG/EORTC) in 16% and as pneumonitis grade I–II in10%. There were no differences in toxicity between the two groups. Three- and five-year overall survival was 25% and 16%, respectively. Median survival was 20.8 months with no significant difference between the two groups. In conclusion, TRT with a total dose of 60 or 45 Gy is feasible with comparable toxicity and no difference in local control or survival. Distant metastasis is the main cause of death in this disease; the future challenge is thus further improvement of the systemic therapy combined with optimised local TRT.
During the last century there was a change in treatment strategies for small cell lung cancer limited-disease (SCLC-LD), beginning with surgery, followed by radiation therapy (RT) and finally chemotherapy (CHT). Further progress was achieved when CHT and RT were combined, resulting in improved overall survival as described in two meta-analyses by Warde and Pignon in 1992 Citation[1], Citation[2]. However, the optimal combination of these two treatment modalities is still not clear. There are many factors to be taken into account when combining CHT and RT in the treatment of SCLC, e.g. timing of RT (early vs. late), target absorbed dose, fractionation (conventional vs. accelerated) and target volumes for the thoracic RT. These issues have been the subjects of many studies aimed at establishing the optimal treatment for SCLC-LD Citation[3–10].
Prophylactic cranial irradiation (PCI) was introduced, with favourable effects according to two large meta-analyses Citation[11], Citation[12], due to the high risk of brain metastasis in this patient group.
Based on these results, a new regional treatment protocol was designed for SCLC-LD, in western Sweden. It consisted of platinum-based CHT in combination with accelerated RT at two different dose levels, 60 Gy and 45 Gy, the latter for patients with large tumour burdens and/or impaired pulmonary function. TRT was administered concurrently with the second or third course. The detailed treatment protocol is presented elsewhere.
The primary aim of this retrospective study was to analyse the outcome of this treatment protocol, including overall survival, local control, feasibility and toxicity. The secondary aims were to study weather the total radiation dose was of importance, as well as to identify prognostic factors for therapy outcome.
Methods
During the period January 1998–December 2004, all patients with SCLC-LD were identified and treated according to the new treatment protocol consisting of chemotherapy with carboplatin (AUC 5, according to Calvert's formula, day 1) and etoposide (100 mg/m2, day 1–3 i.v., or 120 mg/m2 day 1–5 per os) as soon as possible after diagnosis. The choice of carbo- instead of cisplatin was based on the difference in toxicity and the lack of evidence of difference in efficacy Citation[13–15]. TRT was given as 3D-CRT on linear accelerators with 6 MV photons. Patients were immobilised in vacuum pillows and a therapeutic computed tomography (CT) of the chest was performed. Planning target volume (PTV) was defined as the persisting tumour volume after previous chemotherapy as visualised on the therapeutic CT, with a 1.5–2.0 cm margin. Dose plans generally consisted of 3–5 fields and isocentric treatment technique was used. The fractionation was 1.5 Gy BID, with at least six hours between fractions. The planned dose was 60 Gy during 4 weeks but patients with large tumour burdens, poor lung function or impaired general condition were given 45 Gy, according to the clinician's judgement. Approximately one third of the patients were given 60 Gy to macroscopic tumour with margin and had an adjuvant target volume, including the mediastinal lymph nodes, treated to 45 Gy. In the following analyses the whole group of patients given 60 Gy is analysed together. TRT was administered concurrently with the second or third cycle of carboplatin/etoposide. Chemotherapy treatment continued after the TRT with the aim of administering a total of six cycles.
Treatment evaluation after end of therapy included chest x-ray, CT of the chest and brain and, for the majority of patients, a bronchoscopy. If there was a complete response (CR) or “good” partial response (PR), PCI was administered as follows: 2 Gy, once daily, 5 days a week to a total of 30 Gy. Patients were followed up every 3 months with chest x-ray or CT for the first 2 years and subsequently every 6 months.
Data concerning age, sex, tumour stage, Karnofsky performance status, lung function, chemotherapy, number of CHT cycles, irradiated volume, toxicity, PCI, response, local control and cause of death have been retrospectively compiled.
Statistics
The overall survival rates were analysed according to the Kaplan Meier method and are measured from the date of diagnosis. Possible differences between comparable groups, for example males and females, were examined by log-rank test. Possible differences between non-comparable groups were examined by cox-regression test where confounding factors were taken into account. This method was used concerning the groups receiving different dose levels. Univariate and multivariate analyses for possible prognostic factors were performed.
Results
Patient characteristics
The whole material consisted of 80 patients. The median age was 65, ranging from 38 to 83, and there was a slight predominance of women (56%). Patients were given an average of 5.6 cycles of chemotherapy and the TRT was administered concurrently with the third CHT cycle (range 2–6). Thirty-four patients received 45 Gy, for the reasons mentioned previously, and 46 patients were given 60 Gy. The two groups (60 and 45 Gy) are presented in . In addition to differences in tumour stage (TNM) and pulmonary function, patients also differed with respect to Karnofsky performance status and irradiated volume; the high-dose group had a better performance status and smaller treated volumes. This group also included more patients with N0 disease, fewer females and the patients were slightly younger than in the 45 Gy group. Both groups were given the same number of chemotherapy cycles.
Table I. Characteristics of the two dose groups.
Radiotherapy-related acute toxicity
The major acute side effect was esophagitis: 15% of the 45 Gy group and 17% of the 60 Gy group suffered grade 3 esophagitis, as defined by the EORTC/RTOG (requires i.v. nutrition or nasogastric tube). Regarding pulmonary reactions, only 11% contracted some kind of pneumonitis with a maximum severity of grade 2. No grade 4 or 5 radiotherapy-related acute toxicity occurred.
Radiotherapy-related late toxicity
The prevalence of late lung toxicity with fibrosis and impaired lung function is hard to estimate as it has not been specifically studied. One patient in the 60 Gy group had a late esophageal stenosis.
Response
Evaluation of treatment response by chest x-ray and CT, as described above, was based on the RECIST criteria Citation[16]. Assessment of treatment response by chest x-ray might be obscured by radiotherapy-induced fibrosis. In the high-dose group there was 33% CR, 58% PR, 0% stable disease (SD) and 9% progressive disease (PD). The corresponding figures in the lower-dose group are 35% CR, 55% PR, 0% SD and 10% PD, indicating no significant difference in treatment response between the two groups.
We did not observe any significant difference in local control, defined as freedom from progression on CT or chest x-ray at last follow-up. Local control was maintained in 70% of the high-dose group and 65% of the low-dose group.
Survival
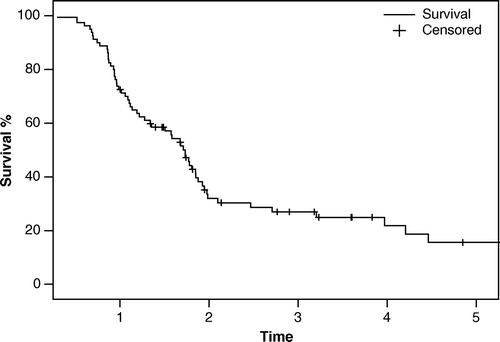
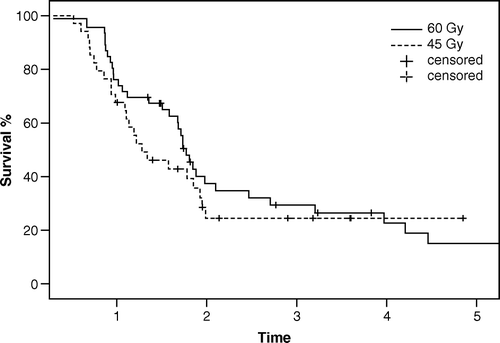
The overall survival was analysed using the Kaplan-Meier method and is shown in . The 3-year and 5-year survival was 25% and 16%, respectively, with a median survival of 20.8 months. The survival for the two different dose levels is shown in . A cox-regression analyse integrating five confounding factors (sex, lung function, PTV, performance status and age) did not show any survival difference between the groups. The median follow-up was 36 months (range 10–72).
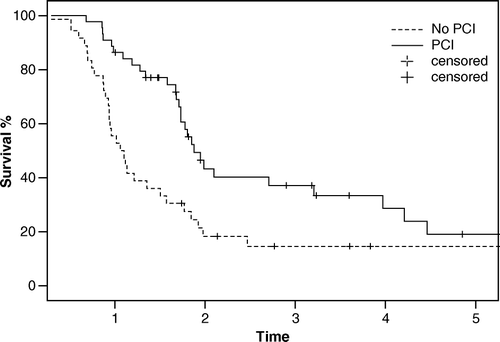
We also analysed potential prognostic factors for survival. The only significant variable found was the administration of PCI; the 3- and 5-year survival was 39% and 20% vs. 15% and 15%, respectively (p = 0.002). However, the reason for this is obvious as only good responders after primary treatment were given PCI ().
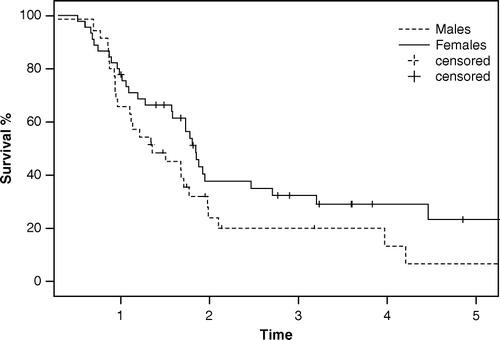
We did observe trends towards improved survival in patients with better lung function (higher FEV1%), patients given radiotherapy early in the treatment regimen, patients in whom local control was achieved after completion of the therapy and patients with N0 disease. The latter group is, however, rather small in SCLC. It is notable that seven of eight patients with N0 disease were given 60 Gy. There was a clear trend towards improved survival in females (3- and 5-year survival 33% and 24%, respectively) compared with males (20% and 7%, respectively), but this difference did not reach significance (p = 0.09) (). We did not observe any significant differences after univariate or multivariate analyses regarding age and number of chemotherapy cycles.
Cause of death
The two groups did not differ substantially regarding cause of death; the main cause was distant metastasis, especially brain metastases (). No treatment related deaths were observed in either group.
Table II. Cause of death in the two dose groups.
Discussion
CHT in combination with TRT is considered to be standard treatment for SCLC-LD Citation[1], Citation[2]. Factors such as timing and sequencing of TRT, fractionation, optimal doses of both CHT and TRT and treatment volumes to be irradiated, are among the important topics attaining much interest in clinical SCLC research Citation[17], Citation[18].
Several studies have focused on the question of TRT timing. The data are somewhat contradictory, but two meta-analyses show a significant difference between early vs. late TRT Citation[4], Citation[10]. This effect was more evident for accelerated and hyperfractionated therapy combined with platinum-based chemotherapy. Regarding sequential or concurrent TRT, there seems to be an advantage to protocols with concurrent therapy Citation[3], Citation[19], Citation[20].
Is fractionation of importance in SCLC? Initial studies from 1999 showed a benefit from accelerated treatment Citation[6], Citation[8], but no difference was found in a comparison between treatment once or twice daily in a meta-analysis the same year Citation[7]. Bonner et al. concluded that when TRT is delayed until the fourth EP cycle, irradiation twice daily did not result in improvement of local control or survival, compared to once daily Citation[21]. However the previously mentioned meta-analysis by Fried et al. established the superiority of accelerated hyperfractionated RT Citation[4].
Concerning total dose, a study by Coy et al. compared two dose levels, 25 Gy vs. 37.5 Gy, showing a significantly improved local control in the higher-dose group, but no difference in survival Citation[9]. A study comparing TRT regimens, 30 Gy (2 Gy once daily) administered to patients with extensive disease and 60 Gy to patients with LD, also found improved local control in the high-dose group Citation[22]. A recent phase II study by Bogart et al. showed that 70 Gy with conventional fractionation is feasible in SCLC-LD Citation[23].
The majority of the questions mentioned above were addressed when a new treatment programme for patients with SCLC-LD was introduced in western Sweden in 1997. Many clinicians consider the administration to this patient population of 1.5 Gy BID to a total of 60 Gy during 4 weeks, combined with chemotherapy, as impossible and associated with unacceptable toxicity. However, our results in the 80 patients treated according to this protocol indicate that it is indeed feasible. The main toxicity problem is grade 3 esophagitis, afflicting 15% and 17%, respectively, in the two dose groups, an acceptable rate, in our opinion, when appropriate supportive care is offered to the patients. The 3- and 5-year overall survival – 25% and 16%, respectively – is comparable with results of the majority of modern SCLC-LD studies Citation[24]. Overall survival is quite similar in the two groups, although there is a negative selection in the lower-dose group. No substantial difference regarding local control was observed either. A possible explanation for this is the relatively small number of treated patients. Our low-dose group (45 Gy BID) received a higher biological dose than in other comparative studies in which the low-dose groups were given about 25–30 Gy Citation[9], Citation[22]. However, it might well be that there is no dose-response relationship in SCLC-LD at doses above 45 Gy BID.
The trend towards improved survival observed in patients given early concurrent treatment is in accordance with previously published data. We did not have an upper age limit for inclusion; the oldest patient treated was 83 years old. In this analysis, age was not found to be a significant prognostic factor and older patients benefit from this treatment similarly to the younger population. Age has been reported to be an uncertain criterion for outcome and tolerability of treatment in SCLC-LD Citation[25].
PCI is beneficial to responding patients and yields a statistically significant survival advantage, even in this relatively small material in which a 3- and 5-year survival of 39% and 20% vs 15 and 15%, respectively, was found. The reason for this is obvious, however, as only good responders after primary treatment were given PCI.
Distant metastasis is the main problem in SCLC and the main cause of death (about 70%) in our study. New chemotherapeutic agents and novel treatment approaches are under intensive investigation. Encouraging results have been reported, among others, by Arriagada and Thatcher who have shown that slightly higher chemotherapy doses and the supplement of an additional chemotherapeutic agent lead to significantly improved survival Citation[26], Citation[27]. Even if the frequency of distant relapse can be reduced, local control is a prerequisite for long-term survival and it is therefore necessary to optimise local treatment as much as possible. This retrospective study has shown that it is possible to escalate the local absorbed dose and use an alternative fractionation regimen in combination with early administration of modern combination chemotherapy in the treatment of SCLC-LD, with acceptable toxicity. We consider these results to be important when new multi-national trials for further optimisation and standardisation of radiotherapy for SCLC-LD are designed.
References
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992; 10: 890–5
- Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, et al. A meta-analysis of thoracic radiotherapy for small cell lung cancer. N Engl J Med 1992; 327: 1618–24
- Catane R, Lichter A, Lee YJ, Brereton HD, Schwade JG, Glatstein E. Small cell lung cancer: Analyses of treatment factors contributing to long term survival. Cancer 1981; 48: 1936
- Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited stage small cell lung cancer. J Clin Oncol 2004; 22: 4837–45
- Murray N, Coy P, Pater J, Hodson I, Arnold A, Zee BC, et al. Importance of timing of thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. J Clin Oncol 1993; 11: 336
- Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 340: 265–71
- Cancer Care Ontario. The role of thoracic radiotherapy as an adjunct to standard chemotherapy in limited stage small cell lung cancer: Practice guidelines report 7-3-3, 1/03 update. http://www.cancercare.on.ca/pdf/full7_13_3.pdf.
- Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small-cell lung cancer: A randomised study. J Clin Oncol 1997; 15: 893–900
- Coy P, Hodson I, Payne DG, Evans WK, Feld R, MacDonald AS, et al. The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Int J Radiat Oncol Biol Phys 1988; 14: 219–26
- De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, Kester A, Rutten I, Lambin P. Systemic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage small-cell lung cancer. Ann Oncol 2006; 17: 543–52
- Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ. Prophylactic cranial irradiation for patients with small cell lung cancer in complete remission. N Eng J Med 1999; 341: 476–84
- Meert AP, Paesman M, Berghmans T, Martin B, Mascaux C, Vallot F, et al. Prophylactic cranial irradiation in small cell lung cancer: A systemic review of the literature with meta-analysis. BMC Cancer 2001; 1: 5
- Skarlos DV, Samantas E, Kosmidis P, Fountzilas G, Angelidou M, Palamidas P, et al. Randomised comparison of etoposide-cisplatin versus etoposid-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group Study. Ann Oncol 1994; 5: 601–7
- Lassen U, Kritjansen K, Osterlind K, Bergman B, Sigsgaard TC, Hirsch FR, et al. Superiority of cisplatin or carboplatin in combination with teniposide and vincristine in the induction chemotherapy of small-cell lung cancer. A randomised trial with 5 years follow up. Ann Oncol 1996; 7: 365–71
- Kosmidis PA, Samantas E, Fountzilas G, Pavlidis N, Apostolopoulou F, Skarlos D. Cisplatin/etoposide versus carboplatin/etoposide chemotherapy and irradiation in small cell lung cancer: A randomized phase III study. Hellenic Cooperative Oncology group for Lung Cancer Trials. Semin Oncol 1994; 21: 23–30
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–16
- Faivre-Finn C, Lorigan P, West C, Thatcher N. Thoracic radiation therapy for limited-stage small cell lung cancer: Unanswered question. Clin Lung Cancer 2005; 7: 23–9
- Faivre-Finn C, Lee LW, Lorigan P, West C, Thatcher N. Thoracic radiotherapy for limited-stage small cell lung cancer: Controversies and future developments. Clin Oncol 2005; 17: 591–8
- Stuschke M, Pöttgen C. Localized small cell lung cancer: Which type of thoracic radiotherapy and which time schedule. Lung cancer 2004; 45: 133–7
- Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002; 20: 3054–60
- Bonner JA, Sloan JA, Shanahan TG, Brooks BJ, Marks RS, Krook JE, et al. Phase III comparison of twice-daily split course irradiation versus once-daily irradiation for patients with limited stage small-cell lung carcinoma. J Clin Oncol 1999; 17: 2681–91
- Papac RJ, Son Y, Bien R, Tiedemann D, Keohane M, Yesner R. Improved local control of thoracic disease in small cell lung cancer with higher dose thoracic irradiation and cycle chemotherapy. J Radiat Oncol 1987; 13: 993–8
- Bogart JA, Herndon II JE, Lyss AP, Watson D, Miller AA, Lee ME, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of cancer and leukaemia group B study 39808. Int J Rad Oncol Biol Phys 2004; 59: 460–8
- Janne PA, Freidlin B, Saxman S, Johnson DH, Livingston RB, Shepherd FA, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 2002; 95: 1528–38
- Weinmann M, Zimmermann F, Bamberg M, Jeremic B. Curative approaches to lung cancer in the elderly. Semin Surg Oncol 2003; 21: 182–9
- Arriagada R, Le Chevalier T, Pignon JP, Riviere A, Monnet I, Chomy P, et al. Initial chemotherapeutic doses and survival in patients with limited small-cell lung cancer. N Engl J Med 1993; 329: 1848–52
- Thatcher N, Qian W, Clark PI, Hopwood P, Sambrook RJ, Owens R, et al. Ifosfamide, carboplatin and etoposide with mid-cycle vincristine versus standard chemotherapy in patients with small cell lung cancer and good performance status: Clinical and quality-of-life results of the British Medical Research Council multicenter randomized LU21 trial. J Clin Oncol 2005; 23: 8371–9