To the Editor
Overexpressed human epidermal growth factor receptor 2 (HER2) is found in 25–30% of breast cancer patients, who have poorer prognosis with shorter overall survival and disease-free survival Citation[1], Citation[2]. The introduction of a monoclonal antibody – trastuzumab (Herceptin®, F. Hoffmann-La Roche, Basel, Switzerland) – as a form of targeted therapy in breast cancer patients with visceral disease, especially in combination with cytostatic drugs, has improved the results of palliative treatment in this patient group Citation[2]. Unfortunately, the concentration of trastuzumab in cerebrospinal fluid is low and, therefore, significant regression of visceral disease and, parallel to it, progression of the disease within the central nervous system (CNS) has been frequently observed Citation[3]. In view of these facts, early recognition of cerebral metastases and the immediate application of palliative radiotherapy of the brain could delay life-threatening neurological complications and maybe even increase overall survival Citation[2], Citation[4].
The aims of this study were to evaluate the frequency of occult brain metastases in patients with breast cancer and overexpressed HER2 currently treated with cytostatic drugs and trastuzumab due to breast cancer dissemination, and to assess the radiological response to palliative whole-brain radiotherapy (WBRT) in these patients.
The pilot study was performed at the Department of Breast Cancer at the Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology in Warsaw, Poland between October and December 2004. Thirty-two breast cancer patients with distant metastases and/or locoregional failure and overexpressed HER2, currently treated in the clinic, were enrolled into the study in order to perform screening MRI of the brain during the asymptomatic period. Median age at the development of the disease was 52 years and the majority of patients were in stage II or III of primary clinical advancement. In 14 patients the tumour cells were oestrogen- and/or progesterone-receptor positive; in the remaining 18 patients they were negative. The time from initial diagnosis to recurrence of the disease was between 0 and 122 months (median: 32 months). In 27 patients there were distant metastases (lung, liver or bone metastases) with or without locoregional failure, while five patients had only locoregional failure. At the time of the study, the patients were being treated with trastuzumab and cytostatic drugs due to disseminated disease. Fifteen patients received first-line chemotherapy and the remaining 17 patients received second- and third-line chemotherapy. Many cytostatic drugs were used, among which the most common were paclitaxel (nine patients), vinorelbine (16 patients) and cisplatin (ten patients). These drugs were administered individually or in combination, but always together with trastuzumab. All the patients were free from any neurological symptoms, both subjectively and on physical and neurological examination, and all underwent MRI screening of the brain. Time between diagnosis of recurrence (distant metastases, locoregional failure) and brain MRI was 3–84 months (median: 15 months).
Occult brain metastases were found in 11 of the 32 patients (34%): five patients had a single lesion and six patients had multiple small, disseminated lesions. The size of the metastatic lesions varied between 2 and 50 mm; no patients had oedematous zones around the lesions. The median time between diagnosis of disease recurrence and the discovery of brain metastases was 15 months.
In order to evaluate the risk factors of brain metastases development, Cox's multivariate analysis was performed, with the following results: patient age at diagnosis, p = 0.70; stage of primary advancement, stage II p = 0.32, stage III p = 0.76, stage IV p = 0.54; time to recurrence, p = 0.55; type of recurrence-distant metastases with or without local recurrence vs. local recurrence p = 1.0; type of recurrence – visceral metastases (lungs/liver) vs. other type of relapse p = 0.19. Based on these results, we failed to identify a subgroup of patients at significant risk of developing brain metastases.
Whole brain radiotherapy (WBRT) was performed in patients with occult brain metastases: a total dose of 30 Gy was administered in 10 fractions of 3 Gy. Irradiated patients underwent a second MRI scan 3 months after the completion of radiotherapy in order to analyse regression of the lesions. WBRT was performed in ten of 11 patients with occult brain metastases because one patient, with a single metastatic lesion in the brain, died before the onset of radiotherapy due to rapid visceral disease progression. In a control MRI scan performed 3 months after the completion of WBRT, three patients had complete remission (CR) and seven patients had partial remission (PR). All patients were free from any clinical neurological symptoms. The initial MRI brain scan an the response to radiotherapy in 4 cases are presented on to .
Figure 1. Coronal T1W contrast-enhanced image. Patient 1. a) Magnetic resonance imaging scan of solitary occult subtentorial brain metastasis before radiotherapy. b) Complete remission 3 months after whole-brain radiotherapy.
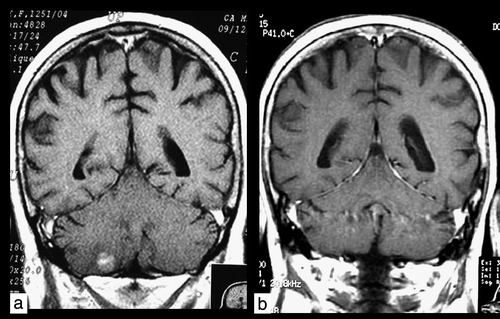
Figure 2. Coronal T1W contrast-enhanced image. Patient 2. a) Magnetic resonance imaging scan of occult multiple subtentorial and supratentorial metastases before radiotherapy. b) Partial remission 3 months after whole-brain radiotherapy.
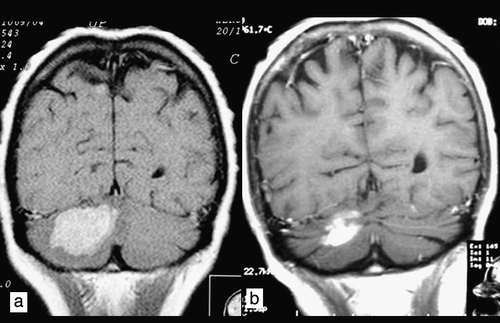
Figure 3. Coronal T1W contrast-enhanced image. Patient 3. a) Magnetic resonance imaging scan of multiple occult subtentorial and supratentorial metastases before radiotherapy. b) Partial remission 3 months after whole-brain radiotherapy.
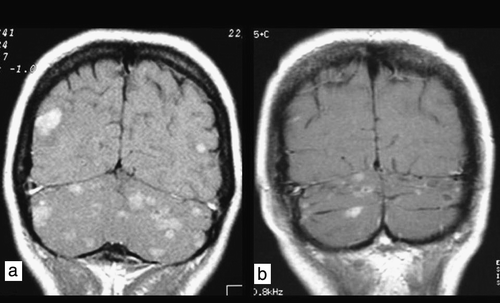
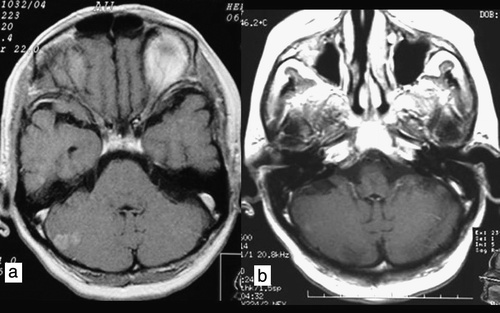
Figure 4. Axial T1W contrast-enhanced image. Patient 4. a) Magnetic resonance imaging scan of multiple ethereal metastases without adenomatous zones around the metastatic lesions. b) Partial remission 3 months after whole-brain radiotherapy.
Based on the literature, in the course of breast cancer, symptomatic brain metastases are observed in 6–16% of patients Citation[3] and in patients with HER2-overexpressing tumours – in 24–48% Citation[1–9]. Brain metastases in patients with overexpressed HER2 are significantly more common in younger women (aged < 50 years) Citation[1], Citation[10] with visceral disease Citation[1], Citation[3], Citation[6], a good response to systemic treatment Citation[3] and short recurrence-free survival Citation[10]. While the results of our study suggested that patients with occult brain metastases were younger, with shorter time to recurrence and more often with visceral metastases, Cox's multivariate analysis failed to identify any risk factors for the development of brain metastases. However, this may be a result of the small patient numbers.
We have found no reports concerning the efficacy of irradiation in occult brain metastases. However, some authors have suggested that in patients with overexpressed HER2 and visceral disease, especially those with a good response to palliative treatment with cytostatic drugs and trastuzumab, early diagnosis of brain metastases and immediate palliative irradiation appear to be specifically advisable Citation[2], Citation[4]. On the other hand, at present there seems to be no evidence that the early detection of brain metastases alters survival or quality of life in patients with breast cancer, although no prospective studies have specifically addressed this issue. In view of the lack of data concerning the efficacy of WBRT of occult brain metastases, examination of larger group of patients and longer follow-up is necessary in order to observe the dynamics of the clinical and radiological changes within the CNS and to discover the causes of death among these particular patients. If further MRI scans reveal rapid progression of the brain metastases and a majority of the patients die because of neurological complications despite the early onset of radiation therapy, then early routine extent-of-disease diagnostics, including screening MRI scans may well be omitted. If, however, the improvement in quality of life or overall survival is observed, early detection of brain metastases would be purposeful and routine MRI should be advised as an element of staging in all breast cancer patients with overexpressed HER2 in stage IV of advancement.
Overall in our study, the incidence of occult brain metastases detected in women with metastatic breast cancer was higher than was expected and the response to WBRT evaluated 3 months after the completion of treatment was good. The results of this preliminary study lie behind the design of the second stage, which commenced in December 2004. The aim of the second stage includes evaluating the time lapse between the first signs of dissemination and the appearance of occult brain metastases, the evaluation of the evolution of brain metastases on the basis of MRI scans performed every 3 months, the length of survival with brain metastases and the cause of death. The results shall be known soon.
Conflicts of interest
Anna Niwińska, Malgorzata Tacikowska and Tadeusz Pienkowski had no financial support or potential conflicts of interest.
References
- Lai R, Dang CT, Malkin MG. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 2004; 101: 810–6
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003; 97: 2972–7
- Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer 2004; 40: 379–82
- Herrero A, Grandez R, Puertolas T, Orduna VA, Trufero JM, Cid RP, et al. High incidence of brain metastases at the time of death in women with metastatic breast cancer treated with trastuzumab. Proc Am Soc Clin Oncol 2004; 23: 67, (abs 765)
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 from metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92
- Yau T. Brain as a sanctuary site for early relapse in patients with advanced breast cancer treated with trastuzumab. Breast 2005; 14((Suppl 1))S43, (abs P101)
- Lai RK, Dang C, Thaler H, Malkin M. The incidence of brain metastases in HER2/neu+ stage IV breast cancer patients. Proc Am Soc Clin Oncol 2001; 20: 66b, (abs 2014)
- Wardley AM, Danson S, Clayton AJ, Clemons M, Burt P, Stewart A, et al. High incidence of brain metastases in patients treated with trastuzumab for metastatic breast cancer at a large cancer center. Proc Am Soc Clin Oncol 2002; 21: 61a, (abs 241)
- Weitzen R, Zach L, Kaufman B, Tichler T, Rath P, Pfeffer J, et al. High incidence of brain metastasis (BM) in patients on trastuzumab (H) for advanced breast cancer. Proc Am Soc Clin Oncol 2002; 21: 31b, (abs 1936)
- Altaha R, Crowell E, Hobbs G, Higa G, Abraham J. Increased risk of brain metastases in patients with HER-2/neu-positive breast carcinoma. Cancer 2005; 103: 442–3
