Abstract
Background and purpose. In patients with locally advanced rectal cancer, neoadjuvant long course (45–54 Gy in 25–30 fractions) chemoradiotherapy (CRT) may reduce tumour size and result in downstaging. In patients with primary resectable tumour short course (25 Gy in 5 fractions) radiotherapy (SCRT) reduces local recurrence but downstaging the disease or altering tumour size has not been described. We aimed to assess change in tumour size on MRI after SCRT or CRT.
Material and methods. Nineteen patients with rectal carcinoma underwent MRI before and after SCRT or CRT. In each case, tumour length and width were documented and number of locoregional lymph nodes recorded. Total mesorectal excision was performed in 15 patients and MR findings correlated with histopathology.
Results. Ten patients received SCRT and nine CRT. Tumour length reduced by 19% overall (15% following SCRT, 23% following CRT). Significant reduction in overall tumour thickness of 27% was seen (25% following SCRT, 29% following CRT). Greater than 30% reduction (partial response) in maximum tumour thickness was seen in 4/10 (40%) following SCRT and 5/9 (56%) following CRT.
Conclusions. Significant reduction in tumour size can be achieved with preoperative long course CRT and SCRT. This unexpected finding following SCRT has not been previously described.
Local recurrence of carcinoma of the rectum results in significant and disabling symptoms with limited palliative treatment options and poor survival. Recurrence rates of up to 40% were previously reported following conventional surgery with poor 5 year disease free survival of around 44% Citation[1–3]. The subsequent introduction of total mesorectal excision (TME) surgery has led to a significant reduction in local recurrence rates. TME involves the en block dissection of the rectum and surrounding mesorectum containing vessels, nodes and lymphatics. TME surgery alone has been shown to reduce local recurrence to around 5% Citation[4–6] and is now the widely accepted surgical practice for operable rectal carcinoma.
The Swedish Rectal Cancer Trial Citation[7] demonstrated a 58% reduction in local recurrence rates with the addition of preoperative short course radiotherapy (25 Gy in 5 fractions over 5 days) to conventional surgery, as compared to surgery alone (27% recurrence versus 11%). Recurrence rates can be reduced further to below 3% with combined TME surgery and short course radiotherapy Citation[8].
In patients with locally advanced rectal carcinoma, preoperative long course radiotherapy (45–54 Gy in 25–30 fractions over 5–6 weeks) with concomitant chemotherapy is well recognised to reduce tumour size and can result in downstaging. In contrast, the benefit of short course radiotherapy on local control is thought to be due to a sterilising effect on the tumour and surrounding vascular and lymphatic drainage pathways rather than by reducing tumour bulk. In this prospective observational study we investigated the direct effect of short course radiotherapy (SCRT) and long course chemoradiotherapy (CRT) on rectal tumour size and local lymph nodes using MRI before and after treatment.
Materials and methods
Study population
Study approval was granted by the local research ethics committee. Thirty-seven consecutive patients with biopsy-proven adenocarcinoma of the rectum who were referred for preoperative MRI were initially enrolled in the study. Of these, four patients underwent palliative treatment only for widespread metastatic disease at presentation and were subsequently excluded, as was one patient who refused radiotherapy. One patient who was unable to tolerate MRI due to claustrophobia and a further 12, for whom imaging with MRI was either unobtainable or incomplete, were excluded. The remaining 19 patients were included in the study; 14 male and five female with a median age of 62 years (range 34 to 82 years). The management plan for each patient was determined by consensus agreement at local Colorectal Multidisciplinary Team meetings. Ten patients received SCRT, eight had long course CRT but one failed to complete CRT, receiving only 7 fractions plus chemotherapy. All 19 patients underwent pre- and post-radiotherapy MRI. Post-treatment MRI scans were performed a mean of 28.1 days (median 35 days) after CRT and 1.3 days (median 0 days) after SCRT. Eighteen patients went on to TME surgery. Surgery was performed a mean of 50.7 days (median 48 days) after completion of CRT and 7.3 days after SCRT (median 6.7 days). In one patient laparotomy revealed widespread locally invasive disease (T4) and surgery was abandoned.
Radiation technique
In all cases the clinical target volume for radiotherapy included the primary tumour, mesorectum and internal iliac nodes below the sacral promontory. The volume was localised on a CT-based planning system and patients were treated using a 3- or 4- field technique using at least 6 MV photons. The prescribed dose was specified according to the International Commission on Radiation units and Measurements 50 guidelines. For SCRT a dose of 25 Gy in 5 fractions of 5 Gy was delivered over 5–6 days. TME surgery was scheduled within 7 days of completion of the radiotherapy. For CRT a dose of 45 Gy in 25 fractions of 1.8 Gy was delivered over 5 weeks, treating daily, Monday to Friday. Concomitant chemotherapy comprised either continuous infusion of 5-Fluororacil at a dose of 200 mg/m2 body surface area per 24 hours or oral Capecitabine at a dose of 750–825 mg/m2 body surface area twice daily. In all cases the chemotherapy was given continuously (including weekends) throughout the radiotherapy. TME surgery was scheduled for 6–12 weeks after completion of the CRT.
Imaging technique
MRI of the rectum was obtained before and after SCRT or CRT using a 1.5T magnet (Signa Horizon, General Electric Medical Systems, Milwaukee, WI). Thin section T2 weighted (T2W) fast spin-echo images were obtained in sagittal, axial and axial-oblique planes (perpendicular to the long axis of the tumour) with the following parameters; Sagittal T2W; TR/TE 5000/135, 3 mm slice thickness with 0.3 mm interslice gap, 38 cm field of view (FOV), 4 acquisitions, 512×224 matrix; axial T2W, TR/TE 5000/135, 8 mm slice thickness with 2 mm interslice gap, 38 cm FOV, 2 acquisitions, 512×256 matix; axial oblique small FOV T2W; TR/TE 4000/105, 3 mm slice thickness with no interslice gap, 24 cm FOV, 3 acquisitions, 512×256 matrix. Axial T1 weighted (T1W) spin echo images performed with TR/TE 400/15, 8 mm slice thickness with 2 mm interslice gap, 24 cm FOV, 2 acquisitions and 256×256 matrix.
Measurements
Measurements of tumour length and width were made: a single length measurement was made on the T2W sagittal images; a total of five tumour width measurements were taken. For this, five measurements were made from the radial margin of the tumour to the luminal surface and their sum calculated (sum of longest diameters; sum LD), as shown in . Comparison of pre- and post-radiotherapy MRI scans was made and width measurements taken at matched levels (). All visible locoregional nodes were documented for size and position. The total number of visible nodes before and after radiotherapy was documented.
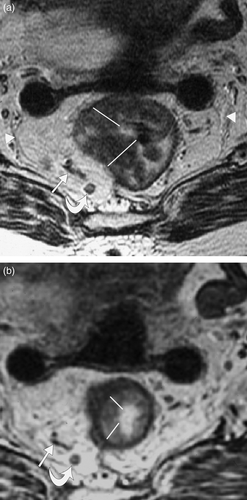
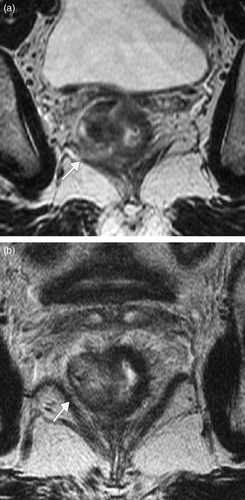
Figure 1. T2 weighted MRI images obtained perpendicular to the long axis of the rectal tumour (a) before and (b) after short course radiotherapy showing reduction in tumour width post radiotherapy. In this case, tumour width reduced by 52% following short course radiotherapy. The images are obtained are at exactly matched levels as shown by the position of an adjacent lymph node (curved arrow) and vessels (arrow). The markers show how multiple radial measurements of tumour thickness were obtained to calculate the overall width measurement as a sum of the longest diameters (sum LD) according to RECIST criteria. The position of the mesorectal fascia is shown (arrow heads).
Statistical analysis
A measure of significance of change in tumour length and width was calculated using the 1-sample student's t test comparing pre radiotherapy length or width (sum LD) with change in size. Significance in change of number of visible locoregional nodes before and after radiotherapy was made using the Wilcoxon signed rank sum test.
Results
Tumour size
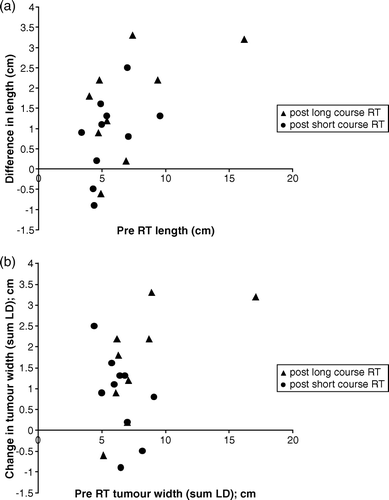
Reduction in tumour width and length was observed following both SCRT and CRT ( and ). The decrease in length and width for individual tumours are shown as a scatter chart in . There was a significant reduction in tumour length from a mean of 5.6 cm to 4.7 cm following SCRT (p = 0.028); and from 7.1 cm to 5.5 cm following CRT (p = 0.006). Overall, preoperative treatment reduced tumour length by 19% with a 15% reduction after SCRT and 23% after CRT. Similarly, significant reduction in tumour width was observed in both treatment groups. The sum LD reduced from a mean of 6.5 cm to 4.9 cm following SCRT (p = 0.002) and from 8.1 cm to 5.7 cm following CRT (p = 0.001). This was equivalent to 29% overall reduction in width post-treatment, (25% reduction following SCRT and 29% following CRT). We used the RECIST Citation[9] criteria as a guide to describe the change in tumour size as measured by the change in the sum LD. A greater than 30% reduction in mean tumour thickness (partial response) was seen in 4/10 (40%) patients following SCRT and 5/9 (56%) following CRT.
Figure 2. Sagittal T2 weighted MRI through rectal tumour (a) before and (b) after short course radiotherapy showing reduction in tumour length post treatment. In this case, tumour length reduced by 33% following short course radiotherapy.
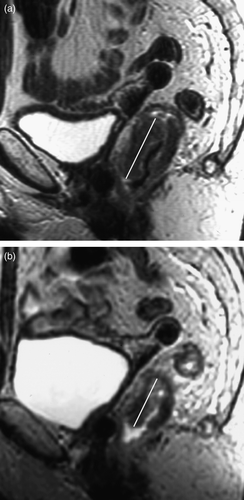
Figure 3. Scatter charts showing reduction in tumour (a) length and (b) width for each individual patient as shown by change in tumour (a) length and (b) width (y-axis) plotted against pre treatment (a) length and (b) width (x-axis). All points above the x axis are those which reduced in size. Those on or below the x axis either did not change or demonstrated a measurable increase in size.
Nodes
The total number of visible loco-regional lymph nodes significantly reduced from 106 to 74 nodes (31%; p < 0.05) following long course radiotherapy. The total number of visible nodes reduced from 62 to 56 following short course radiotherapy (10%) but this was not statistically significant (p > 0.05).
Discussion
MRI has gained widespread acceptance in rectal cancer imaging in recent years and is now the primary tool for preoperative staging. High staging accuracies of 55–100% have been reported Citation[10–15]. The development of the pelvic phased array coil has resulted in high spatial resolution imaging of the pelvis Citation[16], Citation[17] and enables exquisite depiction of the mesorectal fascia (mrf), which is the surgical plane for TME surgery. The circumferential resection margin (crm) is the depth of tissue between the edge of the tumour and the mrf at histology, and needs to be a minimum of 1 mm to ensure safe and complete resection. Demonstrating the relationship between extramural tumour and the mrf is vital, as microscopic involvement of the resection margin predicts local recurrence rates of up to 85% Citation[18]. Preoperative MRI predicts surgical margin involvement with over 90% accuracy Citation[19] when the distance between the edge of the tumour and mrf measures 1 mm or less. Close agreement between MRI measurements and histopathological measurement of the crm have been shown Citation[15], Citation[19]. The absence of crm involvement at histology can be predicted when the distance between the edge of the tumour and the mrf measures more than 1 mm on MRI, with a negative predictive value of up to 93% Citation[14], Citation[20].
Tumour downstaging is well recognised following neoadjuvant preoperative CRT for locally advanced rectal carcinomas. These tumours are not amenable to primary curative surgery and CRT is given with the aim of shrinking the tumour to a more favourable and operable stage. Tumour downstaging by preoperative CRT has been reported in up to 41% of cases Citation[21]. Tumour downstaging increases with dose although higher fractions are also associated with increased complication rates and a standard dose of 45 Gy is given. Preoperative CRT has also been used in the treatment of resectable rectal cancer, where it has been shown to improve local control in comparison to postoperative chemoradiotherapy, with reduced toxicity Citation[22]. Our findings support the concept that CRT has a downstaging effect on both the primary tumour and locoregional nodes.
Short course radiotherapy (SCRT), given as 25 Gy over 5 days with TME surgery performed shortly after, has been shown to reduce local recurrence rates to below 3% at 2 years Citation[8]. However, in this setting, improvement in local control does not appear to relate to downstaging of the irradiated tumour and nodes Citation[23]. The benefit of preoperative radiotherapy in reducing local recurrence rates has been further supported by a large meta-analysis of 22 randomised trials by the Colorectal Cancer Collaborative Group Citation[24]. Concerns have been raised of the increased risk of late toxicity with SCRT and modifications to the traditional 5×5 Gy treatment schedule may address this issue, as recently discussed in Acta Oncologica Citation[25].
Our study gives the first direct demonstration of reduction in rectal tumour bulk following short course radiotherapy. Despite the relatively small number of patients, this reduction reaches statistical significance for both tumour length and width and is seen despite an anticipated increase in tumour size resulting from radiation-induced oedema. By obtaining measurements of tumour width in exactly the same plane and at carefully matched levels, we are confident we have directly observed downsizing of the tumour. The change in tumour volume was an immediate effect and cannot be attributed to a delay in post-radiotherapy imaging. The mean time from the end of SCRT to post treatment MRI was just 1.3 days (median 0 days). Our results are consistent with the reduction in tumour size after SCRT inferred by a large study by Graf et al. Citation[26] who observed significantly smaller tumours on the pathological specimens of 471 patients who received preoperative SCRT compared with 235 patients who received TME surgery alone. They also observed a lower percentage of higher stage tumours in the irradiated versus non-irradiated group (33% and 42% respectively for Dukes C tumours), leading investigators to infer a downstaging effect of SCRT. Similarly, The Swedish Rectal Cancer Trial Citation[7] also suggested downstaging following short course radiotherapy when they observed significantly more Dukes stage A and B tumours in patients randomised to radiotherapy plus surgery compared with those who received surgery alone (68% vs. 59%; p = 0.008).
In a more recent study by Bujko et al. Citation[27] patients with resectable clinical stage T3 and T4 tumours were randomised to preoperative short course radiotherapy or chemoradiotherapy. They found similar rates of survival and local control in both groups. The equally beneficial effects of both treatments maybe due to tumour downstaging, further suggested by the finding of pathological stage T1 and T2 tumours in 39.5% of the short course group following resection. Interestingly, apparent downstaging from T2/early T3 to T1 was observed in one patient in our study following SCRT (). In a second patient, tumour was seen to touch the potential surgical resection margin on the pre-radiotherapy MRI but is clear of it following SCRT, and appears to convert an ‘at risk’ resection margin to a safe resection margin ().
Figure 4. Oblique axial T2 weighted MRI through rectal tumour (a) before and (b) after short course radiotherapy. (a) Tumour invasion was thought to be just through the muscle layer (stage T3) with loss of normal low signal muscle wall (arrows). (b) Following short course radiotherapy the low signal inner and outer muscle layers (arrows) are now visible (stage T2 on MR but T1 on histology), suggesting downstaging.
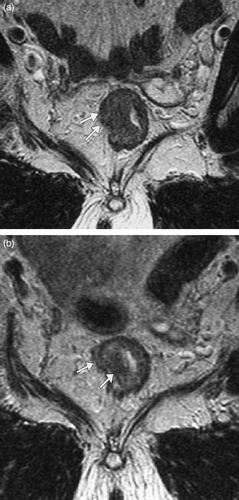
Figure 5. Oblique axial T2 weighted MRI through a low rectal tumour in a different patient (a) before and (b) after short course radiotherapy. (a) Tumour is seen to abut, possibly invade the right levator plate initially (arrow) with an ‘at risk’ crm. (b) Following short course radiotherapy there is a clear fat plane between the tumour (with intact muscle layer) and the levator muscle (arrow) and the crm is preserved.
Pathological grading of the degree of tumour regression following preoperative chemoradiotherapy, based on the ratio of residual tumour to fibrosis, has been described Citation[28] and applied to rectal carcinoma. Tumour regression scores (TRG) equating to major tumour regression and have been reported in 59–73% of patients following CRT Citation[29], Citation[30]. In addition, a more favourable outcome in terms of overall and disease free survival is associated with major tumour regression compared to non-responders, suggesting TRG to be an independent predictor of outcome Citation[29], Citation[31]. The results of our study are supported further by the finding of major pathological tumour regression in 21% of patients following short course radiotherapy in one study Citation[30]. Our study has provided a direct demonstration of tumour regression before surgery and could be taken forward to assess the prognostic implications of preoperative imaging on tumour reduction.
A number of clinical and pathological factors predict tumour responsiveness to preoperative radiotherapy. These include radiation dose, preoperative clinical stage, tumour differentiation and time before surgery Citation[32]. Higher rates of tumour regression or downstaging associated with a longer time interval between the end of radiotherapy and surgery have been observed Citation[32], Citation[33]. Dahl et al. Citation[33] compared their findings of complete tumour regression rate of 4.5% following preoperative radiation dose of 31 Gy with 2.6% regression rate in the EORTC study Citation[34] where the time interval between radiotherapy and surgery was 2–3 weeks in the prior study versus median 11 days in the later. We found no correlation between reduction in tumour size and time interval from radiotherapy to surgery for either SCRT or CRT in our study but the number of cases is small. It is possible that sequential MRI following CRT will demonstrate continued tumour response with time in some cases and might be used to optimise the timing of surgery.
Tumour related factors which may predict responsiveness have also been suggested. One study examining the ratio of p53 and p21 tumour suppressor gene expression in rectal carcinoma patients found those with mutated wild-type p53 expression to be significantly more radioresponsive Citation[35]. Research in tumour cell kinetics may help in patient selection for adjuvant treatment in the future by predicting radioresponsiveness, although as yet, no specific markers exist.
In conclusion, we report a significant decrease in the size of rectal carcinoma following both short and long course radiotherapy. This is an unexpected finding following SCRT. Future developments in the use of MRI in predicting tumour responsiveness to therapy may provide prognostic information and help tailor individual treatment.
References
- Malcolm AW, Perencevich NP, Olson RM, Hanley JA, Chaffey JT, Wilson RE. Analysis of recurrence patterns following curative resection for carcinoma of the colon and rectum. Surg Gynecol Obstet 1981; 152: 131–6
- Rao AR, Kagan AR, Chan PM, Gilbert HA, Nussbaum H, Hintz BL. Patterns of recurrence following curative resection alone for adenocarcinoma of the rectum and sigmoid colon. Cancer 1981; 48: 1492–5
- Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer 1983; 52: 1317–29
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1(8496)1479–82
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341(8843)457–60
- Moore E, Heald RJ, Cecil TD, Sharpe GD, Sexton R, Moran BJ. Almost all five year disease free survivors are cured following rectal cancer surgery, but longer term follow-up detects some late local and systemic recurrences. Colorectal Dis 2005; 7: 403–5
- Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336:980–7.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345: 638–46
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16
- McNicholas MM, Joyce WP, Dolan J, Gibney RG, MacErlaine DP, Hyland J. Magnetic resonance imaging of rectal carcinoma: A prospective study. Br J Surg 1994; 81: 911–4
- Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: Thin-section MR imaging for staging in 28 patients. Radiology 1999; 211: 215–22
- Wallengren NO, Holtas S, Andren-Sandberg A, Jonsson E, Kristoffersson DT, McGill S. Rectal carcinoma: Double-contrast MR imaging for preoperative staging. Radiology 2000; 215: 108–14
- Kim NK, Kim MJ, Park JK, Park SI, Min JS. Preoperative staging of rectal cancer with MRI: Accuracy and clinical usefulness. Ann Surg Oncol 2000; 7: 732–7
- Beets-Tan RG, Beets GL, Borstlap AC, Oei TK, Teune TM, von Meyenfeldt MF, et al. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI?. Abdom Imaging 2000; 25: 533–41
- Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001; 357(9255)497–504
- Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol 2005; 78(927)245–51
- Brown G, Kirkham A, Williams GT, Bourne M, Radcliffe AG, Sayman J, et al. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am J Roentgenol 2004; 182: 431–9
- Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986; 2(8514)996–9
- Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 2003; 90: 355–64
- Blomqvist L, Rubio C, Holm T, Machado M, Hindmarsh T. Rectal adenocarcinoma: assessment of tumour involvement of the lateral resection margin by MRI of resected specimen. Br J Radiol 1999; 72(853)18–23
- Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum 2002; 45: 895–903
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- Marijnen CA, Nagtegaal ID, Klein KE, Hermans J, van de Velde CJ, Leer JW, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol 2001; 19: 1976–84
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358(9290):1291–304.
- Glimelius B. Rectal cancer irradiation. Long course, short course or something else?. Acta Oncol 2006; 45: 1013–7
- Graf W, Dahlberg M, Osman MM, Holmberg L, Pahlman L, Glimelius B. Short-term preoperative radiotherapy results in down-staging of rectal cancer: A study of 1316 patients. Radiother Oncol 1997; 43: 133–7
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–23
- Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73: 2680–6
- Bouzourene H, Bosman FT, Seelentag W, Matter M, Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 2002; 94: 1121–30
- Vironen J, Juhola M, Kairaluoma M, Jantunen I, Kellokumpu I. Tumour regression grading in the evaluation of tumour response after different preoperative radiotherapy treatments for rectal carcinoma. Int J Colorectal Dis 2005; 20: 440–5
- Wheeler JM, Dodds E, Warren BF, Cunningham C, George BD, Jones AC, et al. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: Correlation with rectal cancer regression grade. Dis Colon Rectum 2004; 47: 2025–31
- Berger C, de Muret A, Garaud P, Chapet S, Bourlier P, Reynaud-Bougnoux A, et al. Preoperative radiotherapy (RT] for rectal cancer: Predictive factors of tumor downstaging and residual tumor cell density (RTCD]: Prognostic implications. Int J Radiat Oncol Biol Phys 1997; 37: 619–27
- Dahl O, Horn A, Morild I, Halvorsen JF, Odland G, Reinertsen S, et al. Low-dose preoperative radiation postpones recurrences in operable rectal cancer. Results of a randomized multicenter trial in western Norway. Cancer 1990; 66: 2286–94
- Gerard A, Berrod JL, Pene F, Loygue J, Laugier A, Bruckner R, et al. Interim analysis of a phase III study on preoperative radiation therapy in resectable rectal carcinoma. Trial of the Gastrointestinal Tract Cancer Cooperative Group of the European Organization for Research on Treatment of Cancer (EORTC]. Cancer 1985; 55: 2373–9
- Fu CG, Tominaga O, Nagawa H, Nita ME, Masaki T, Ishimaru G, et al. Role of p53 and p21/WAF1 detection in patient selection for preoperative radiotherapy in rectal cancer patients. Dis Colon Rectum 1998; 41: 68–74
