Abstract
Purpose. Radiation enteritis is the main acute side-effect during pelvic irradiation. The aim of this study was to quantify the dose-volume relationship between irradiated bowel volumes and acute enteritis during combined chemoradiotherapy for rectal cancer.
Material and methods. Twenty-eight patients with locally advanced rectal cancer received chemoradiotherapy. The radiation therapy was given with a traditional multi-field technique to a total dose of 50 Gy, with concurrent 5-Fluorouracil (5-FU) and oxaliplatin (OXA) based chemotherapy. All patients underwent three-dimensional CT-based treatment planning. Individual loops of small and large bowel as well as a volume defined as “whole abdomen” were systematically contoured on each CT slice, and dose-volume histograms were generated. Diarrhea during treatment was scored retrospectively according to the NCI Common Toxicity Criteria scale.
Results. There was a strong correlation between the occurrence of grade 2+diarrhea and irradiated small bowel volume, most notably at doses >15 Gy. Neither irradiated large bowel volume, nor irradiated “whole abdomen” volume correlated significantly with diarrhea. Clinical or treatment related factors such as age, gender, hypertension, previous surgery, enterostomy, or dose fractionation (1.8 vs. 2.0 Gy/fraction) did not correlate with grade 2+diarrhea.
Discussion. This study indicates a strong dose-volume relationship between small bowel volume and radiation enteritis during 5-FU-OXA-based chemoradiotherapy. These findings support the application of maneuvers to minimize small bowel irradiation, such as using a “belly board” or the use of IMRT technique aiming at keeping the small bowel volume receiving more than 15 Gy under 150 cc.
In locally advanced rectal cancer, long-term preoperative radiotherapy is often used with the purpose of shrinking the tumor and thus facilitating surgery Citation[1]. The radiation effect can be potentiated by the use of concomitant chemotherapy Citation[2], Citation[3]. The most frequently used drug in chemoradiotherapy regimens for rectal cancer is 5-fluorouracil (5-FU). In recent years several new agents with activity against colorectal cancer have been introduced. One of these is oxaliplatin (OXA) which significantly enhances the antitumoral activity of 5-FU in patients with advanced colorectal cancer Citation[4], Citation[5]. Preclinical studies have demonstrated that OXA has good radiosensitizing properties Citation[6]. Its toxicity profile makes OXA an attractive drug to combine with 5-FU and abdominal irradiation. For these reasons, OXA is nowadays frequently incorporated in chemoradiotherapy regimens for locally advanced rectal cancer Citation[7], Citation[8]. The main acute toxicity of chemoradiotherapy against rectal cancer is enteritis, with grade 3 + diarrhea occurring in about 11–39% of the cases in 5-FU based chemoradiotherapy Citation[1], Citation[2], Citation[9], Citation[10], and is slightly more frequent than with radiotherapy alone Citation[2].
Factors associated with enteritis in patients receiving radiotherapy include, total dose, fractionation, and irradiated bowel volume. Only few studies have been performed to elucidate the relationship between irradiated bowel volume and treatment induced enteritis during pre- or postoperative chemoradiotherapy for rectal cancer Citation[11–13].
The aim of our investigation was to further analyze the quantitative relationship between irradiated bowel volumes and acute enteritis during 5-FU-OXA based chemoradiotherapy, using information gathered by 3D treatment planning tools from patients treated for locally advanced rectal cancer. Three different normal tissue volumes were analyzed based on dose volume histogram (DVH) data: 1) Small bowel, which is generally considered a major organ at risk (OAR) for radiation-induced diarrhea; 2) Large bowel, which has not previously been linked to development of radiation-induced diarrhea, but should be of interest to study given the fact that inflammation in colonic mucosa often leads to diarrhea; 3) “Whole abdomen”, a volume that would be easier to delineate for treatment planning in clinical practice compared to 1) and 2).
Material and methods
Patient characteristics
Twenty-eight patients (18 men and ten women) with locally advanced adenocarcinoma of the rectum were treated with 5-FU-OXA based chemoradiotherapy at the Department of Oncology, University Hospital of Lund, Sweden during 2000–2003. Median age was 59 years (range 32–78). Twenty patients had primary T4 tumors and the remaining eight had locally recurrent disease. All tumors were truly locally advanced with a median maximal tumor diameter of 7.8 cm (range 5.0–12.0 cm). The median distance from the anal orifice to the lowest part of the tumor was 5.1 cm (range 0–16.0). Tumor involvement of the genitourinary organs (the prostate gland, seminary vesicles, urinary bladder or the uterus), was present in 11 cases. Seventeen of the patients had distant metastases (liver: 10, lung: 3, liver + lung: 3, scrotum: 1). Twelve had colostomy and three had ileostomy. One patient had diabetes mellitus and eight had hypertension needing medication. No patient had any previous history of inflammatory bowel disease.
Chemotherapy
The chemotherapy was given according to the Nordic FLOX regimen Citation[14], with bolus 5-FU and leucovorin, 400 mg/m2–60 mg/m2 i.v., respectively on day 1–2 and OXA infusion 85 mg/m2 i.v. on day 1. This was repeated every 2 weeks during radiotherapy.
Radiotherapy
All patients were treated in a supine position with the arms above the head. A 3D CT based treatment plan was performed for all patients. The abdomen and pelvis were scanned with 10 mm thick abutting slices. No oral contrast was used. The gross tumor volume (GTV) was delineated based on MRI and diagnostic CT-imaging. The GTV was defined as the primary tumor in the rectum and when lymph-node metastases in the pelvis were diagnosed, these were also included in the GTV. The clinical target volume (CTV) was defined as the GTV with approximately 2vcm margins. The planning target volume (PTV) constituted CTV with 5 mm margins to compensate for variations in patient set-up. The first consecutive 13 patients received 50.0 Gy at the ICRU Reference point Citation[15], Citation[16] in 25 fractions with one fraction per day and the following 15 patients 50.4 Gy in 28 daily fractions, i.e. 1.8 Gy/fraction. The reduction of dose per fraction was due to a change in treatment policy at the department, with the intent to decrease toxicity.
Patients with local disease only, received elective pelvic node irradiation using a shrinking field technique, delivering 41–46 Gy to the tumor and the pelvic lymph nodes at risk, followed by a boost to the tumor to a total absorbed dose of 50/50.4 Gy. In patients with distant metastases, no elective irradiation of non-involved lymph nodes was performed. These patients received conformal radiotherapy to the tumor only to an absorbed dose of 50/50.4 Gy. Elective lymph node irradiation was given as follows: perirectal nodes in 26 (of 28) cases, obturator nodes in 24 cases, presacral nodes in 23 cases, internal iliacal nodes in 22 of the cases, external iliacal in five cases and inguinal nodes in one case.
The treatment plans for all 28 patients were calculated and analyzed with the treatment planning system, Helax-TMS (Nucletron B.V., The Netherlands). The typical treatment technique consisted of three MLC-shaped conform fields, one dorsal and two lateral wedged fields using photons with beam energies ≥10 MV. No specific bowel sparing maneuver was applied.
Toxicity scoring
Toxicity data were collected retrospectively from the patient charts. Diarrhea, as a measure of acute enteritis, occurring during the chemoradiotherapy period was chosen as primary endpoint parameter. The degree of diarrhea was scored according to the NCI Common Toxicity Criteria (CTC) scale, version 2.0 Citation[17], see . In most of our analyses diarrhea grade was used as a dichotomized variable, i.e. grade 0–1 vs. 2–4. The reason for this cut-off (and not the commonly used grade 0–2 vs. 3–4) was that also grade 2 diarrhea, allowing up to six extra stools per day or nocturnal stools, is a clinically important toxicity, often leading to treatment modifications.
Table I. NCI CTC grading of diarrhea.
Treatment-plan analysis
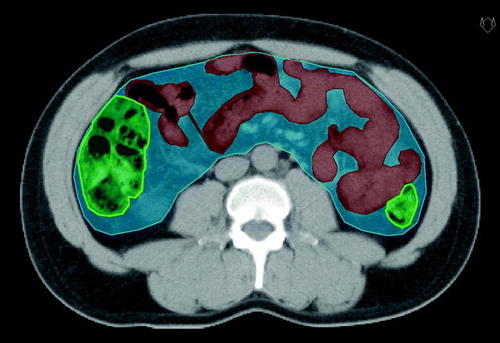
In the treatment planning CT scans, three different volumes of interest were delineated (). 1) Small bowel: the outer contour of every visible small bowel sling. In patients with ileostomy only the “stool-bearing” afferent bowel segments were delineated. 2) Large bowel: the outer contour of every visible colon sling, from ceacum to rectum. In patients with ileostomy there were no “stool-bearing” colon segments to delineate and subsequently no large bowel volume was calculated in these cases. In patients with colostomy, only the “stool-bearing” afferent bowel segments were delineated. 3) “Whole abdomen”: all abdominal content including small bowel, large bowel, mesenteric structures, and abdominal fat. Liver, kidneys, spleen, large vessels, and psoas muscles were excluded. Dose-volume histograms were extracted for the three structures described above and volumes (in absolute and relative numbers) that had received doses larger than 5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 Gy respectively were calculated (i.e. V5, V10,…,V50).
Figure 1. Delineated bowel volumes: 1) Small bowel (red structure), 2) Large bowel (green), and 3) “Whole abdomen” (blue).
The patient material is considered too small for deriving input parameters with any statistical significance for the dose-volume dependence of diarrhea for commonly used NTCP models, e.g. n, m and D50 for the “Lyman-Kutcher-Burman” model Citation[18–20] or s, γ and D50 for the seriality model Citation[21]. The dose-volume dependence can to some extent, however, also be checked with the “generalized concept of Equivalent Uniform Dose”, gEUD Citation[22] defined as1 where N is the number of bins in the differential DVH for the structure of interest and vi and Di are the fractional volume and the total dose deposited in bin i, respectively. The a-value (positive for normal tissues) is an empirical tissue-specific parameter describing the dose-volume dependence of the organ. An a-value equal to the inverse of the volume parameter n in the LKB-model is often used. Parallel arranged tissue structures have a-values close to one, i.e. the toxicity of the organ is well correlated to the mean dose, while organs with a highly serial structure have a-values typically in the range of∼10–20 and with a toxicity correlated to the high dose part of the DVH (EUD is equal to the maximum dose for a=∞).
The dose D in the EUD-equation could also be corrected for fractionation effects by replacing it with e.g. the equivalent dose at 2 Gy, EQD2 using the LQ-formalism,2 where di is the dose per fraction for bin i of the DVH.
gEUD was calculated for a range of a-values between 1 and 20. The calculations were made using the total physical dose D as well as EQD2 (with α/β = 10 Gy for acute effects) in Equation 1 for small and large bowel as well as for “whole abdomen”. The diarrhea scores where dichotomized (grades 0 + 1 vs. grades 2 + 3, respectively) and any differences in EUD between the two groups were analyzed (the Mann-Whitney U-test) with a-values varying in the range mentioned above.
Statistical analysis
All the statistical calculations were performed in Stata 9.2 (Stata Corporation, 2006). For univariate analysis of the association between dichotomized diarrhea grade (0–1 vs. 2–4) and irradiated bowel volume, the Mann-Whitney U-test was chosen whereas Fisher's exact test was used for evaluation of the association between diarrhea and other patient and treatment associated factors. The correlation between diarrhea score 0–3 and other factors of interest were assessed using Spearman's rank correlation coefficient (rs). Multiple linear regression models with diarrhea score as dependent variable were used to adjust the effect of irradiated bowel volume for each of the factors found to have strongest association to diarrhea in univariate analysis. Null hypotheses of differential effect of irradiated volume on diarrhea score in groups defined by other factors were tested by fitting linear models with interaction terms. All tests were two-sided and p-values < 0.05 were considered statistically significant.
Results
General clinical outcome
Sixteen patients received 1–3 cycles of FLOX just before start of chemoradiotherapy. All patients received either two or three chemotherapy cycles during the radiation therapy. Twenty-one (75%) patients received all chemotherapy cycles as planned. In seven (25%) cases, the third cycle was either omitted or given at a reduced dose because of neutropenia (two patients) or enteritis (five patients). Overall seven treatments were delayed due to toxicity, but none more than one week. All patients received full dose radiation therapy as planned with four patients needing breaks in the scheduled radiotherapy (2–9 days) because of gastro-intestinal toxicity.
Determination of the anti-tumor effect of the treatment was not within the scope of this retrospective study. However, all patients were evaluated with a CT scan or an MRI at 4–6 weeks after end of treatment. The response rate (CR + PR) was 64% (18/28). The patients were then referred back to the surgical department. Further treatment and follow-up was up to the discretion of the treating surgeon.
Toxicity
Diarrhea during chemoradiotherapy occurred as follows: 10, 7, 3, 8, and 0 patients had grade 0, 1, 2, 3, and 4, respectively. The median radiation dose at which diarrhea grade 2–3 occurred was 30 Gy (mean 27 Gy). Other grade 3 toxicities were two cases of nausea, three cases of anorexia, and six cases of neutropenia. Ten patients were admitted to the hospital due to toxicity. The only grade 4 toxicities were two cases with neutropenia. No cases of grade 3+ polyneuropathy, anemia or stomatitis were observed and no treatment related deaths occurred.
Normal tissue irradiation and diarrhea
In the univariate analysis we found that patients with diarrhea grade 2+ had on average larger absolute volumes, VX, of irradiated small bowel for all cut-off doses, X, studied. The differences were highly significant for cut-off doses X > 15 Gy as shown in a and . The correlation between diarrhea grade 0–3 and irradiated small bowel volume was also high (rs=0.60–0.64) with p-values < 0.001 for all doses in the interval from >15 Gy to >45 Gy. Similar results were obtained when relative small bowel volumes were analyzed in the same manner but the p-values were an order of magnitude higher, but still significant for all cut-off doses.
Figure 2. Dose-volume histograms for small bowel (a), large bowel (b), and “whole abdomen” (c), divided by diarrhea grade 0–1 vs. grade 2–3. Dots indicate volumes of normal tissue in individual patients receiving >5, >10, >15,…,>50 Gy. Lines indicate median volumes as a function of dose level. Mann-Whitney U-test was used.
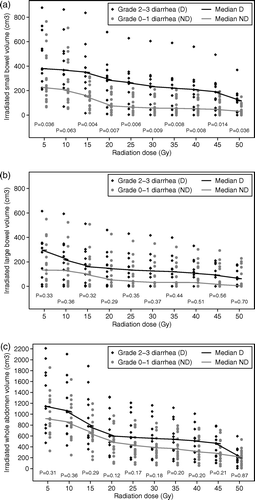
Patients with diarrhea grade 2+ also had higher VX values for the large bowel and “whole abdomen” compared to patients with grade 0–1 diarrhea, but the difference was not statistically significant for any dose level, b and c, respectively.
Patients with grade 2+ diarrhea were given significantly higher equivalent uniform doses (gEUD) to the small bowel compared to patients with grade 0–1 diarrhea. This was true for all a-values, irrespectively if D or EQD2 was used in Equation 1. The strongest significance was found for a = 1.0 (p = 0.003). The corresponding analysis for large bowel and “whole abdomen” revealed no statistically significant differences for any a-value.
Other potential risk factors
The relationship between several other clinical features and diarrhea are presented in . None of the factors investigated were significantly associated with the risk of developing grade 2+ diarrhea. However, diarrhea grade 0–3 was found to be correlated to the absence of enterostomy (rs=0.36; p = 0.06) and also weakly to locally recurrent disease (as opposed to primary tumor; rs=0.27; p = 0.17). The correlation between diarrhea grade 0–3 and small bowel volume receiving >25 Gy, the cut-off with the highest correlation to diarrhea grade, was found to be significant also after adjustment for each of these two factors, one at a time, in multiple linear regression models, p = 0.001 in both models. Also the other dose cut-offs in the range from >15 Gy to >45 Gy lead to significant correlation after adjustment (p ≤ 0.002).
Table II. Association between grade 2 + diarrhea and patient and treatment associated factors using Fisher's exact test.
No significant interaction effects were seen, but the correlation between irradiated volume and diarrhea score was found to be higher in the subgroup with enterostomy (p interaction = 0.10; dose cut-off >25 Gy).
Discussion
In locally advanced rectal cancer, combined chemoradiotherapy is often used. The main acute toxicity of this treatment is acute radiation enteritis, mainly manifested as diarrhea. In order to be able to minimize enteritis during pelvic chemoradiotherapy, it is important to identify risk factors associated with it. The radiation dose to the small bowel has long been suggested as a major causal factor for radiation-induced diarrhea, even though there is relatively sparse data supporting it in the literature. Our study confirms this hypothesis, showing a highly statistically significant relationship between irradiated small bowel volume and chemoradiotherapy induced enteritis for patients with locally advanced rectal cancer. Patients suffering from grade 2 + diarrhea had significantly larger irradiated small bowel volumes, with radiation doses larger than 15 Gy as the most significant dose level. This finding is in concordance with previous studies, especially the work of Baglan et al. Citation[12]. Baglan et al. found that no patients with small bowel volume < 150 cc at dose level > 15 Gy had grade 3 + diarrhea as opposed to 50% of those with ≥150 cc. Our results were very similar, showing clinically significant diarrhea in 52% of patients with >15 Gy in >150 cc small bowel, but in only 11% of cases with >15 Gy in ≤150 cc small bowel (). This suggests that one should strive at keeping the small bowel volume that receives >15 Gy lower than 150 cc during the dose-planning process.
Table III. Number of patients with diarrhea grade 0–1 and grade 2–3, respectively, related to whether more or less than 150 cc of the small bowel volume received >15 Gy, p = 0.049 (two sided Fischer's exact test).
Irradiation of the large bowel, on the other hand, is not generally considered a significant risk factor for radiation-induced enteritis, but evidence is largely lacking. No studies on this correlation, using 3D dose-planning tools have been published to our knowledge. We found no significant correlation between irradiated large bowel volume and diarrhea. The reason for this is unclear. One might speculate that it could be due to the fact that a smaller proportion of the colon is being irradiated, or its smaller surface area per length of bowel sling or that it is more radioresistant.
Delineating every bowel sling is very time consuming and difficult in clinical practice. Identification of an easier-to-use substitute volume would be clinically desirable. A concept of “the volume potentially containing small bowel tissue” has been used by others to create dose-volume relationships for organs at risk Citation[23], Citation[24]. We defined a volume called “whole abdomen”. Despite greater “whole abdomen” volumes at each dose level for patients suffering from grade 2 + diarrhea, this difference was not statistically significant. This underlines the importance of retaining detailed information of the actual small bowel volume, and that this easier approach unfortunately did not provide sufficient information.
The patient material is too small for deriving input data for NTCP models and hence to better quantify the dose-volume dependence. Increased knowledge of the dose-volume dependence of the small bowel could clarify whether, for example, the mean dose or the maximum dose is more important in the pathogenesis of radiation induced diarrhea. Our gEUD calculations resulted in significant differences for small bowel between patients with grade 2–3 diarrhea versus those with grade 0–1 but without being able to extract a specific a-value. The strongest significance (p = 0.003) was found for a = 1.0 which could be interpreted as an indicator of a more parallel structure. The results from the same gEUD calculations for large bowel and “whole abdomen” showed that these did not differ significantly.
This is the first study looking at this relationship when OXA has been added to 5FU and radiation for locally advanced rectal cancer. Whether the addition of OXA increases the enteritis is unclear, but our study suggests that it does not seem to alter the volume-dependent correlation.
Besides small bowel irradiation, several other factors may potentially contribute to development of diarrhea, but none of the patient- or treatment related factors investigated in this study were significantly associated with enteritis as shown in . Neither did any of those factors interfere with the major finding of this study, thus indicating that small bowel irradiation indeed is an independent risk factor for radiation enteritis.
It is commonly believed that previous abdominal surgery is associated with an increased risk for radiation enteritis Citation[25]. Our study supports such a hypothesis. Diarrhea grade 2 + occurred in 63% of the patients treated for a local recurrence and in 30% when treated for a primary rectal cancer (). This difference was not statistically significant, whereas the small bowel radiation was significantly associated with enteritis. This could indicate that the commonly observed increase in radiation enteritis in patients that had undergone previous pelvic surgery could be explained by the fact that small bowel slings are attached to pelvic structures due to the surgical trauma, which in turn increases the small bowel volume within the pelvic radiation fields.
It is well known that the fractionation schedule affects the normal tissue damage caused by radiation. An increased dose per fraction enhances the late effects such as fibrosis and telangiectasias, but also to some extent the acute radiation damage. In the present study half of the patients received 2.0 Gy per fraction and the remaining half got 1.8 Gy per fraction. Grade 2 + diarrheas () occurred more frequently in the higher than in the lower fractionation group. However, this difference was not statistically significant.
The clinical data in the present study was gathered retrospectively and the toxicity scoring may therefore be less precise than in a prospective analysis. On the other hand, at our department these patients are routinely followed up by a doctor's visit every week during the whole chemoradiotherapy session, to actively monitor side-effects. Therefore, we feel that the toxicity grading in this study was sufficiently accurate.
The patient population in this study is relatively small, but so are the few previous studies on this subject Citation[11–13]. Despite the limited sample size, the most important results from our study were in good accordance with earlier studies, which increases the reliability of our findings.
In summary, this study confirms that small bowel irradiation is an independent and important risk factor for developing enteritis during chemoradiotherapy for rectal cancer, whereas irradiation of large bowel seems to be of lesser importance in this respect. This is clinically useful information that can be used in the treatment planning process, which primarily should aim at minimizing the small bowel irradiation with the volume receiving over 15 Gy not exceeding 150 cc. One way to achieve this is to treat the patient in a prone position and use a belly board, which has been shown in several previous studies to significantly reduce small bowel volume during pelvic irradiation Citation[26–28]. Another possibility is to use IMRT (intensity modulated radiotherapy).
Acknowledgements
This research is supported by the Gunnar Nilsson cancer foundation, the Berta Kamprad cancer foundation and the Lund University donation funds. Special thanks to Lidia Karolak at the Department of Radiation Physics, Lund University Hospital, for all help with treatment planning.
References
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- Bosset JF, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P, et al. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: Assessment of acute toxicity and treatment compliance, report of the 22 921 randomized trial conducted by the EORTC radiotherapy group. Eur J Cancer 2004; 40: 219–24
- Brændengen M, Tveit KM, Berglund Å, Birkemeyer E, Frykholm G, Påhlman L, et al. A randomized phase III study (LARCS) comparing preoperative radiotherapy alone versus chemoradiotherapy in non-resectable rectal cancer. ECCO 13-the European Cancer Conference 2005;[Abstract #612].
- De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47
- Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan P, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000; 18: 136–47
- Källström J, Kjellén E, Johnsson A. In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol 2005; 44: 687–93
- Rödel C, Grabenbauer GG, Papadopoulos T, Hohenberger W, Schmoll H-J, Sauer R. Phase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancer. J Clin Oncol 2003; 20: 3098–104
- Machiels JP, Duck L, Honhon B, Coster B, Coche J-C, Scalliet P, et al. Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: The radioxcape study. Ann Oncol 2005; 16: 1898–905
- Grann A, Feng C, Wong D, Saltz L, Paty PP, Guillem JG, et al. Preoperative combined modality therapy for clinically resectable UT3 rectal adenocarcinoma. Int J Radiat Oncol Biol Phys 2001; 40: 515–22
- Hyams DM, Mamounas EP, Petrelli N, Rockette H, Jones J, Wieand S, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum. A progress report of the National Surgical Adjuvant Breast an Bowel Project Protocol R-03. Dis Colon Rectum 1997; 40: 131–9
- Minsky BD, Conti JA, Huang Y, Knopf K. Relationship of acute gastrointestinal toxicity and the volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol 1995; 13: 1409–16
- Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2002; 52: 176–83
- Tho LM, Glegg M, Paterson J, Yap C, MacLeod A, McCabe M, et al. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: Investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys 2006; 66: 505–13
- Sørbye H, Glimelius B, Berglund Å, Fokstuen T, Tveit KM, Brændegen M, et al. Multicenter phase II study of Nordic fluorouracil and folinic acid bolus schedule combined with oxaliplatin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2004; 22: 31–8
- International Commission of Radiation Units and Measurements. ICRU report 50: Prescribing, recording and reporting photon beam therapy. Bethesda: International Commission of Radiation Units and Measurements; 1993.
- International Commission on Radiation Units and Measurements. ICRU report 62: Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50). Bethesda: ICRU; 1999.
- Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: Impact on radiotherapy. Int J Radiat Oncol Phys 2000; 47: 13–47
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985; 8: S13–S19
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. Int J Radiat Oncol Biol Phys 1989; 16: 1623–30
- Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991; 21: 123–35
- Källman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992; 62: 249–62
- Niemierko A. A generalized concept of Equivalent Uniform Dose (EUD). Med Phys 1999; 26: 1101
- Muren LP, Jebsen N, Gustafsson A, Dahl O. Can dose-response models predict reliable normal tissue complication probabilities in radical radiotherapy of urinary bladder cancer? The impact of alternative radiation tolerance models and parameters. Int J Radiat Oncol Biol Phys 2001; 50: 627–37
- Muren LP, Hafslund R, Gustafsson A, Smaaland R, Dahl O. Partially wedged beams improve radiotherapy treatment of urinary bladder cancer. Radiother Oncol 2001; 59: 21–30
- Touboul E, Balosso J, Schlienger M, Laugier A. Radiation injury of the small intestine. Radiobiological, radiopathological aspects; risk factors and prevention. Ann Chir 1996; 50: 58–71
- Koelbl O, Richter S, Flentje M. Influence of patient positioning on dose-volume histogram and normal tissue complication probability for small bowel and bladder in patients receiving pelvic irradiation: A prospective study using a 3D planning system and a radiobiological model. Int J Radiat Oncol Biol Phys 1999; 45: 1193–8
- Shanahan TG, Mehta MP, Bertelrud KL, Buchler DA, Frank LE, Gehring MA, et al. Minimization of small bowel volume within treatment fields utilizing customized “belly boards”. Int J Radiat Oncol Biol Phys 1990; 19: 469–76
- Huh SJ, Lim DH, Ahn YC, Kim DY, Kim MK, Wu HG, et al. Effect of customized small bowel displacement system in pelvic irradiation. Int J Radiat Oncol Biol Phys 1988; 40: 623–7
