Abstract
Aim. The current prospective study sought to trace the incidence and severity of oxaliplatin-induced peripheral neuropathy (OXLIPN) and to determine its clinical and electrophysiological pattern. Patients and methods. Twenty-five adult patients scheduled to be treated with 12 courses of the oxaliplatin-based regimen, FOLFOX-4, for metastatic colon cancer participated in this study. Patients were clinically and electrophysiologically monitored at baseline and followed-up during chemotherapy. The severity of OXLIPN was summarized by means of a modified Total Neuropathy Score (TNS). Results. Evidence of OXLIPN was disclosed in 16 of the 25 patients (64%). The mean TNS values for patients manifesting some grade of OXLIPN were 13.9±5.8 (range 7–28). All longitudinal comparisons concerning the motor conduction parameters failed to reach significance. By contrast, comparisons of the median changes at baseline and each of the follow-up studies revealed significant decrease in all sensory action potentials examined. Conclusion. Our results indicate that the majority of patients treated with the FOLFOX-4 regimen would manifest an axonal, predominately sensory peripheral neuropathy, of mild to moderate severity.
Colorectal cancer (CRC) is one of the most frequent types of solid malignancies in the general population Citation[1]. 5-fluorouracil (5-FU) has become the standard chemotherapeutic agent used for treating CRC Citation[2]. Attempts to further improve its clinical results have introduced combination protocols with 5-FU plus Leucovorin (LV) plus Irinotecan (IRI) or Oxaliplatin (OXL). Combination regimens including IRI/LV/5-FU or OXL/LV/5-FU have demonstrated substantial cytotoxic synergy and efficacy in the treatment of patients with advanced or metastatic CRC Citation[3], Citation[4].
OXL, a third generation platinum derivate, is capable of altering the voltage-gated sodium channels, thereby inducing two clinically distinct forms of peripheral neuropathy (PN). The acute syndrome is consisted of distal or perioral paresthesias and pharyngolaryngeal dysethesias. It appears soon after the administration of OXL, is transient and completely reversible within hours or days Citation[3]. The other form of oxaliplatin-induced peripheral neuropathy (OXLIPN) is a chronic pure sensory, axonal neuropathy, closely resembling the cisplatin-induced peripheral neuropathy Citation[5]. Taking into consideration that high cumulative doses of OXL are strongly associated with occurrence of OXLIPN, IRI/LV/5-FU may be the preferable regimen to avoid significant neurotoxicity associated with OXL/LV/5-FU Citation[4].
The aim of this prospective study was to trace the incidence and severity of chronic OXLIPN and to determine its clinical and electrophysiological pattern. The monitoring of acute OXLIPN was not included among our objectives.
Patients and methods
Patients’ selection
To ensure homogeneity of the study sample, we have solely enrolled patients with colon cancer, scheduled to receive 12 courses of the formal OXL (85 mg/m2)-based regimen, FOLFOX-4. Thus, 25 adult patients with a histologically confirmed diagnosis of colon cancer, 17 males and eight females, aged 64.1±9.3 years, were longitudinally studied in the current setting. To be eligible for enrolment, patients had to be over 18 years of age at recruitment, to have satisfactory liver and renal function, life expectancy of at least 9 months, WHO performance score of 0–1 and be able to fully understand the study information provided by the investigators.
Patients having history of peripheral neuropathy (i.e. hereditary, associated with nutritional agents and paraneoplastic causes) as well as patients with systemic or collagen diseases (i.e., diabetes mellitus, SLE, vasculitis, HIV, alcohol abuse) were excluded. Patients were also excluded if they were not chemotherapy naïve, or if evidence of PN was disclosed at baseline screening.
Clinical and electrophysiological evaluation
All patients enrolled were evaluated at baseline by the same neurologist who performed both clinical and electrophysiological evaluations. The clinical evaluation of OXLIPN was based on the Neurological Symptom Score (NSS) and Neurological Disability Score (NDS) Citation[6]. NSS (ranging from 0 being normal to 17 being most severe affected) assessed the occurrence of autonomic symptoms, muscle weakness (bulbar and/or limbs) or evidence of positive (numbness or pain at any site) and negative (difficulty identifying objects in mouth or hands and unsteady gait) sensory symptoms, scoring as present (1) or absent (0). Clinical signs (i.e., cranial nerves function, muscle strength, tendon reflexes, joint position, pinprick and vibration sensation, etc) were assessed using NDS (ranging from 0 being normal to 292 being most severe affected) scored from 0 (no deficit) to 4 (absence of function/severest deficit). The Functional Grading Scale (FGS) assessed the functional ability, particularly mobility, ranging from 0 (healthy) to 5 (requiring artificial ventilation for at least part of the day) Citation[7].
Standard neurophysiological examination was carried out unilaterally (right side), employing the widely accepted criteria of identification of abnormalities Citation[8]. The distal skin temperature was maintained between 32–34°C. The following parameters were estimated: a) peak to baseline amplitude of compound muscle action potential (a-CMAP), distal motor latency (DML), motor conduction velocity (MCV) and F-wave minimum latency of ulnar and peroneal nerves. b) peak-to-peak amplitude of sensory action potentials (a-SAP) and sensory conduction velocities (SCV) of ulnar (orthodromic technique), sural and superficial peroneal nerves (antidromic technique and proximal segment).
The battery of the clinical and electrophysiological tests described above was repeated after four (OXL dose, 340 mg/m2), eight (OXL dose, 680 mg/m2) and 12 (OXL dose, 1020 mg/m2) courses of chemotherapy. The electrophysiological evaluations both pre- and post treatment were confirmed by an independent senior neurologist who checked both the recordings and results.
The results of the clinical and electrophysiological study were summarized by means of a modified Total Neuropathy Score (TNS), validated by Cornblath et al. Citation[9]. The grading of the OXLIPN severity as mild (1–11), moderate (12–23) and severe (>24) was defined by TNS, corresponding to the WHO grading scales 1–3 for chemotherapy-induced PN Citation[10].
Chemotherapy regimen and dose modification
The formal FOLFOX-4 regimen Citation[11] was administered to our patients, consisting of LV 200 mg/m2 as a 2-hour infusion (d1&d2), followed by 5-FU 400 mg/m2 (d1&d2) as an i.v. bolus. 5-FU 600 mg/m2 was administered afterwards as a 22-hour infusion (d1&d2). OXL (Eloxatin, Aventis-Sanofi) 85 mg/m2 was given as a 2-hour i.v. infusion, concurrent with LV (d1). Therapy was repeated every 2 weeks. Routine antiemetic prophylaxis was also used.
Dose adjustments of the study drugs or treatment delays were calculated according to toxicity grade. Dose modification was determined according to the system showing the greatest degree of toxicity. Toxicities were graded using the National Cancer Institute common criteria, with the only exception being the grading of the OXLIPN severity, which was performed according to the modified TNS.
The OXL dose was to be reduced by 30% for persistent or temporary (at least 14 days) painful paresthesia, dysesthesia or functional impairment. If in spite the 30% dose reduction of OXL, the persistent grade 3 (severe) neurotoxicity persisted, than chemotherapy was to be omitted in subsequent cycles. No prophylactic treatment was given for neurotoxicity.
For diarrhoea, patients were to receive supportive care, as well as intensive treatment with loperamide. For grade 2/3 hematologic toxicity, treatment was to be delayed for 1 week or until hematologic recovery. If recovery was not achieved, the dose levels of OXL and 5-FU would be reduced by 20%. Subcutaneous administration of granulocyte colony-stimulating factor 5 µg/kg/day on 5 consecutive days was permitted at the investigator's discretion. For angina or myocardial infarction, treatment was to be ceased. For grade 3 mucositis, after 1 week delay, the dose of 5-FU was to be reduced by 25%. For any grade 4 toxicity, except gastrointestinal or hematologic toxicity, patients were to be withdrawn from the study.
The study protocol was approved by the Institutional Review Board of Patras Medical School and written informed consent prior to study entry was obtained from all patients.
Statistical analysis
Descriptive statistics were generated for all variables. Median values with range were calculated for each electrophysiological variable. The changes in median electrophysiological scores during chemotherapy were examined using the Wilcoxon Matched-Pairs Signed-Ranks Test. All tests were two-sided and significance was set at p < 0.05. Statistical analyses were performed using the SPSS for Windows (release 10.0; SPSS Inc., Chicago, IL).
Results
Patient characteristics
All patients suffered from metastatic (stage IV) colon cancer. The demographic and clinical characteristics of the sample size are presented in . None of the patients enrolled discontinued treatment due to persistent grade 3 or grade 4 toxicity or for any other reason of early withdrawal. No evidence of lethal event was noted during treatment. All patients received the total of the scheduled 12 cycles of treatment.
Table I. Patients’ baseline and clinical characteristics.
Incidence and severity of OXLIPN
Clinical and/or electrophysiological evidences of OXLIPN were disclosed in 16 of the 25 patients (64%). The mean TNS of patients who manifested some grade of neurotoxicity were 13.9±5.8 (range 7–28). TNS values ranged the severity of OXLIPN as mild (grade 1) in six patients (24%), moderate (grade 2) in eight (32%), while severe (grade 3) neuropathy was revealed in two cases (8%). The majority of patients (12/16) started to develop evidence of OXLIPN between courses 8–12, while the other four patients developed OXLIPN between courses 4–8.
Clinical pattern of OXLIPN
After 12 courses of treatment, the mean values of the Neurological Disability Score were 21.1±17.5, while the mean values of the Neurological Symptom Score were 1.8±0.8. Two of the patients who manifested OXLIPN experienced negative sensory symptoms (difficulty identifying objects in mouth or hands and unsteady gait), while all of them experienced positive sensory symptoms, like numbness or pain at any site.
The six patients with mild (grade 1) OXLIPN complained of distal numbness and/or painful paresthesia limited to fingers/toes. Clinical examination revealed decreased vibration sensation limited to wrist/ankle without suppression of tendon reflexes. Patients with moderate (grade 2) OXLIPN (n = 8) presented positive sensory symptoms in a stocking-and-glove distribution, initially manifested in the lower limbs and progressed to involve the upper limbs. Cranial nerves were spared. Proprioceptive sensory disturbances in a stocking-and-glove distribution, with vibration sensation being more affected and ankle hyporeflexia were also evident. Motor nervous dysfunction was not observed. Severely (grade 3 neurotoxicity) affected patients (n = 2) complained of distal numbness and/or painful paresthesia extended up to the knees/elbows, whilst they had also decreased pin and vibration sensation up to the knees/elbows, ankle areflexia and hyporeflexia elsewhere. Mild to moderate weakness, mainly involving the distal muscles (toe extension and finger abduction muscles) was also evident in those patients. These two severely affected patients completed the study, since evidence of persistent grade 3 neurotoxicity was disclosed only at the last follow-up performed after administration of the scheduled 12 courses of treatment.
FGS scores graded the deterioration of patients’ functional status as grade 1 (minor symptoms, fully capable of manual work) in seven, grade 2 (able to walk > 10 m unaided) in eight, whilst grade 3 impairment was noted (able to walk > 10 m with a walker or support) in one patient, despite dose modification. No evidence of grade 4 or 5 functional disability (bed or chair-bound) was observed.
Electrophysiological pattern of OXLIPN
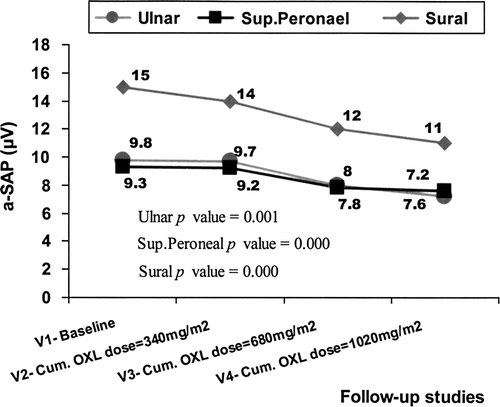
Insignificant changes were encountered after longitudinal comparison of subsequent median scores of the motor conduction parameters during chemotherapy (). By contrast, a significant longitudinal deterioration (baseline vs. after 12 courses of treatment) in the a-SAPs of all three sensory nerves tested was observed (), whereas the subsequent sensory conduction velocities scores were not significantly changed over time (). The needle electromyography (EMG), performed only in patients with severe (grade 3) OXLIPN (n = 2), revealed normal configuration of the motor units without active denervation potentials.
Table II. Longitudinal motor conduction and sensory conduction velocities studies. For each variable the median value-(range) is given.
Discussion
In the current setting, the reported rate (64%) of patients that manifested OXLIPN after administration of the formal FOLFOX-4 regimen is quite similar to that previously reported in several trials Citation[12], Citation[13]. As in most other previous publications Citation[4], Citation[13], the majority of our patients experienced mild or moderate (grade 1–2) OXLIPN. In our study, grade 3 neurotoxicity was observed in 8% of patients, a rate quite similar to that observed in the MOSAIC trial Citation[13], while it is lower than that observed in other studies Citation[5].
Differences in the nature of studies and the methodology applied could account for discrepancies between results. The MOSAIC trial Citation[13], thoroughly monitored both acute and chronic OXLIPN following adjuvant treatment with the FOLFOX-4 regimen, as opposed to our study sample where palliative treatment for metastatic colon cancer was administered and chronic OXLIPN was solely assessed. Additionally, the comparison between our results and those reported in studies using preventive measures against OXLIPN, such as MgCa infusions Citation[14] or carbazepine Citation[15], is difficult mainly due to differences in the applied study design (prospective vs. retrospective and use of FOLFOX-4 vs. FUFOX regimen).
The selective focus on chronic OXLIPN without assessment of acute neuropathy might represent a potential limitation in our study design. In the current setting, we have solely assessed the chronic form of OXLIPN due to the fact that the distal sensory neuropathy related to OXL treatment has the most important clinical implication. On the contrary, the transient acute OXLIPN is usually reversible without persistent impairment of sensory function and in the vast majority of cases does not require discontinuation of treatment or dose modification Citation[16]. Dose reduction and subsequent treatment discontinuation should be only applied in patients with acute neurotoxicity lasting more than 2 weeks, even without motor symptoms or neurological signs Citation[13].
Both the MOSAIC trial Citation[13] and most of all other studies Citation[4], Citation[5] evaluated the efficacy, general tolerability and safety profile of the FOLFOX-4 regimen, being not solely focused on assessing the direct effect of OXL administration on the peripheral nerves. On the contrary, assessment of treatment response (efficacy) during chemotherapy was not included among the objectives of the current setting, since our study has been focused on the detailed neurological monitoring of OXLIPN, being based on validated clinical scales and longitudinal electrophysiological recordings.
The fact that no “oncologic measures” of neurotoxicity, such as the modified Levi scale or the NCI CTC toxicity scale, have been used might as well represent a limitation in the study design, leading to some difficulty to compare our results to other pure oncological publications. However, the incidence and severity of OXLIPN was summarized by means of a modified TNS, previously validated Citation[9] and applied in several studies assessing cisplatin and/or paclitaxel-induced peripheral neuropathies Citation[17–20]. Nevertheless, it is acknowledged that cisplatin and/or paclitaxel neurotoxicities are not quite similar to oxaliplatin neurotoxicity and thus the modified TNS that we used is not validated in oxaliplatin neurotoxicity.
In the current setting, the clinical and electrophysiological examinations pointed towards a diagnosis of a symmetrical, axonal, predominately distal sensory neuronopathy. Positive sensory symptoms in a stocking-and-glove distribution were occurred in the distal lower extremities of our patients. Additionally, the decreased vibration and proprioception as well as the suppression of deep tendon reflexes indicate a dysfunction of sensory nerves Citation[21]. In respect to the electrophysiological findings, a decrease or abolition of a-SAPs, with unchanged sensory conduction velocities was observed. No evidence of distal motor neuropathy was found, thoroughly confirming the predominance of sensory fibres involvement. The blood-nerve barrier is relative more permeable in the dorsal root ganglia (DRG) than elsewhere Citation[22] and this could account for the selective sensory toxicity of platinum compounds, as compared to motor neurons in the anterior horn of the spinal cord.
Considering, that motor nerves are normally spared by the platinum compounds-induced neuropathy Citation[23], needle EMG was not routinely performed in order to avoid discomfort to our patients. However, when performed in the two severely affected cases with clinical weakness no abnormalities were disclosed. The mild muscle weakness observed in those cases, despite the unchanged ulnar and peroneal CMAPs, should be attributed to sensory de-afferentation Citation[24].
In the majority of patients, evidence of OXLIPN occurred initially between chemotherapy courses 8–12. This could be attributed to the OXL doses accumulation, since it is documented that at cumulative doses that reach 800 mg/m2, the occurrence of OXLIPN is highly likely Citation[25]. Severe (grade 3) OXLIPN occurs in 15% after cumulative doses of 750–850 mg/m2 and 50% after a total dose of 1 170 mg/m2 Citation[25].
The mechanisms underlying the acute and chronic OXLIPN have not as yet been clearly defined Citation[25]. Recent data suggest that the acute OXLIPN may be linked to the rapid chelation of calcium by OXL-induced oxalate. Hence, OXL is capable of altering the voltage-gated sodium channels through a pathway involving calcium ions Citation[26]. In view of its clinical similarity with disorders of voltage-gated ion channels such as myotonias, acute OXLIPN could be defined as a channelopathy Citation[27].
On the other hand, the chronic OXLIPN may be induced by the decreased cellular metabolism and axoplasmatic transport resulting from the accumulation of platinum compounds in DRG cells Citation[28]. One could suggest that in addition to morphologic and functional changes in the DRG cells, the prolonged activation of voltage-gated sodium channels could induce cellular stress, thereby affecting the sensory nerve cells. This view is supported by the results of a preclinical study, which reported alterations of sodium channel inactivation kinetics of the sural nerve after application of OXL and increase of sodium currents due to prolonged opening of sodium channels Citation[29]. OXL-induced mitochondrial damage has also been proposed as another potential mechanism of OXLIPN induction Citation[30].
The lack of data concerning the course of OXLIPN after cessation of chemotherapy is a limitation in the current setting. OXLIPN is partly reversible, with a 3 months median time to recovery and completely resolve after 6–8 months in 40% of patients Citation[31]. Hence, long-term longitudinal follow-ups in patients experiencing OXLIPN are needed to obtain data about its reversibility. The assessment of the course of neuropathy after a year of longitudinal follow-up is ongoing in the patients reported herein.
From the clinical point of view, the identification of a specific profile for chronic OXLIPN would be particularly useful for medical oncologists as well as for clinical studies that attempt to identify an ideal neuroprotective agent against OXLIPN. Xaliproden, an experimental non-peptide compound, found in research to have neurotrophic and neuroprotective effects, showed that it is capable to significantly reduce the occurrence of severe OXLIPN Citation[32]. Alternatively, oxcarbazepine, a modern antiepileptic drug that modulates both voltage-sensitive sodium channels and high-voltage activated N-type calcium channels may be another suitable preventive measure against OXLIPN, since recent data are persuasive enough that oxcarbazepine may have a significant neuroprotective effect against chronic OXLIPN Citation[20].
To summarize, our results indicate that the majority of patients with colon cancer treated with the formal FOLFOX-4 regimen would manifest an axonal, predominately sensory peripheral neuropathy, of mild to moderate severity.
Conflicts of interest
We have no conflicts of interest. No funding source had a role in the preparation of this paper or in the decision to submit it for publication.
References
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2003. CA Cancer J Clin 2003; 53: 5–26
- Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: Systematic review and meta-analysis. Colorectal Cancer Collaborative Group. Brit Med J 2000; 321(7260)531–5
- Grothey A, Goldberg RM. A review of oxaliplatin and its clinical use in colorectal cancer. Expert Opin Pharmacother 2004; 5: 2159–70
- Kalofonos HP, Aravantinos G, Kosmidis P, Papakostas P, Economopoulos T, Dimopoulos M, et al. Irinotecan or oxaliplatin combined with leucovorin and 5-fluorouracil as first-line treatment in advanced colorectal cancer: A multicenter, randomized, phase II study. Ann Oncol 2005; 16: 869–77
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47
- Dyck PJ, Thomas PK. Peripheral Neuropathy. 3rd ed. Vol 2. Philadelphia: WB Saunders; 1993. p 1310–7.
- Kleyweg RP, Van der Meché FGA, Schmitz PIM. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve 1991; 14: 1103–9
- Kimura J. Electrodiagnosis in diseases of nerve and muscle, principles and practice. 3rd ed. Oxford: Oxford University Press; 2001. pp. 91–166.
- Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total neuropathy score. Validation and reliability study. Neurology 1999; 53: 1660–4
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- de Gramont A, Bosset JF, Milan C, Rougier P, Bouche O, Etienne PL, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: A French intergroup study. J Clin Oncol 1997; 15: 808–15
- Kemeny N, Garay CA, Gurtler J, Hochster H, Kennedy P, Benson A, et al. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol 2004; 22: 4753–61
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. for the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer. N Engl J Med 2004; 350: 2343–51
- Gamelin L, Boisdron-Celle M, Delva R, Guerin-Meyer V, Ifrah N, Morel A, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: A retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 2004; 10(12 Pt 1)4055–61
- von Delius S, Eckel F, Wagenpfeil S, Mayr M, Stick K, Kullmann F, et al. Carbamazepine for prevention of oxaliplatin-related neurotoxicity in patients with advanced colorectal cancer: Final results of a randomised, controlled, multicenter phase II study. Invest New Drugs 2007; 25: 173–80
- Pasetto LM, D'Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: How and why?. Crit Rev Oncol Hematol 2006; 59: 159–68
- Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann Neurol 1994; 35: 304–11
- Berger T, Malayeri R, Doppelbauer G, Krajnik G, Huber H, Auff E, Pirker P. Neurological monitoring of neurotoxicity induced by paclitaxel/cisplatin chemotherapy. Eur J Cancer 1997; 33: 1393–9
- Argyriou AA, Chroni E, Koutras A, Ellul J, Papapetropoulos S, Katsoulas G, et al. Vitamin E for prophylaxis against chemotherapy-induced neuropathy: A randomized controlled trial. Neurology 2005; 64: 26–31
- Argyriou AA, Chroni E, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T, et al. Efficacy of oxcarbazepine for prophylaxis against cumulative oxaliplatin-induced neuropathy. Neurology 2006; 67: 2253–5
- Peltier AC, Russell JW. Recent advances in drug-induced neuropathies. Curr Opin Neurol 2002; 15: 633–8
- Gregg RW, Molepo JM, Monpetit VJ, Mikael NZ, Redmond D, Gadia M, Stewart DJ. Cisplatin Neurotoxicity: The relationship between dosage, time, and platinum concentration in neurological tissue and morphological evidence of toxicity. J Clin Oncol 1992; 10: 795–803
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 2002; 249: 9–17
- Davar G, Maciewicz RJ. Deafferentation pain syndromes. Neurol Clin 1989; 7: 289–304
- Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer 2005; 5(Suppl 1)S38–S46
- Grolleau F, Gamelin L, Boisdron-Celle M, Lapied B, Pelhate M, Gamelin E. A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channels. J Neurophysiol 2001; 85: 2293–7
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol 2000; 406: 25–32
- Verstappen CC, Heimans JJ, Hoekman K, et al. Neurotoxic complications of chemotherapy in patients with cancer: Clinical signs and optimal management. Drugs 2003; 63: 1549–63
- Adelsberger H, Quasthoff S, Grosskreutz J, et al. Alteration of sodium channel inactivation kinetics on rat sural nerve by the chemotherapeutic agent oxaliplatin. Biol Chem 1999; 380S: 123
- Goodisman J, Hagrman D, Tacka KA, Souid AK. Analysis of cytotoxicities of platinum compounds. Cancer Chemother Pharmacol 2006; 57: 257–67
- Windebank AJ. Chemotherapeutic neuropathy. Curr Opin Neurol 1999; 12: 565–71
- Gibson TB, Ranganathan A, D'Orazio A; American Society of Clinical Oncology. Highlights From: the 2006 American Society of Clinical Oncology Gastrointestinal Cancers Symposium San Francisco, CA, January 2006. Clin Colorectal Cancer 2006;5:398–402.