To the Editor
We report a case of primary signet ring cell carcinoma of the prostate (SRCC) in a 70-year-old healthy, non-smoking man.
History
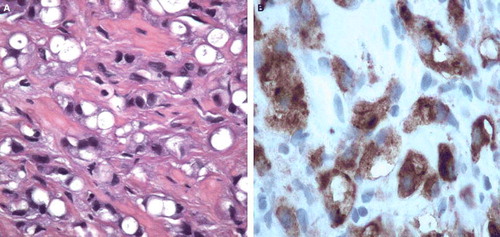
The patient consulted his general practitioner due to pain in the perineum. A blood test was drawn and showed serum prostate specific antigen levels (PSA) of 27 ng/ml. The patient was referred to the local urologist for further investigation. The tumour was regarded as non-organ confined, T3b (TNM-UICC 2002) by digital rectal examination. Transrectally performed ultrasound measured the prostate to 46.5 cm3. Sextant biopsies revealed an adenocarcinoma of low differentiation and signet-ring cell appearance in all six needle biopsies. Gleason grade was 4 + 4 in all samples. The carcinoma cells proved positive for prostate specific antigen (PSA), prostate specific acid phosphatase (PAP) and pancytokeratin (AE1–AE3) (), but did not stain for carcinoembryonic antigen (CEA), cytokeratin 7 and 20, or synaptophysin. Mucin stains were also negative (alcian pH 2.5 or periodic acid-Schiff PAS).
Figure 1. (A) Specimen with HE-staining of tumour cells. (B) Specimen staining positively for PSA and PAP.
The inguinal lymph nodes were resected as part of the staging procedure. Lack of metastases in routine stains was confirmed by negative stains for pancytokeratin (AE1/AE3). Magnetic resonance imaging (MRI) of the vertebral columna and pelvis concluded negative in regard to involvement of the skeletal or the remaining loco-regional lymph nodes.
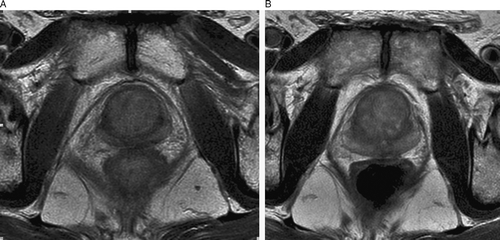
MRI of the prostate was difficult to interpret (). No focal changes were found. A diffuse pattern was interpreted as generalised tumour infiltration of the whole gland, including both seminal vesicles.
Chest x-ray, bone scan, cystoscopy and gastroscopy with biopsies showed no evidence of a metastatic process, and the diagnosis of primary SRCC of the prostate (T3bpN0M0) was confirmed.
Treatment
Neoadjuvant endocrine manipulation with GnRH agonist (goserelin, Zoladex®) combined with definitive radiotherapy (74 Gy) was initiated. Briefly, conformal radiotherapy was applied with a four-field box technique (opposing anterior-posterior fields and two opposing lateral fields). The gross target volume (GTV) was defined by a three-dimensional computed tomography planning system (Oncentra®, Uppsala, Sweden) and covered the prostate and the seminal vesicles. The treatment volume received megavolt photon energy, 15 MV, with daily fractions of 2 Gy, 5 days per week, with a target dose of 74 Gy.
Follow-up
After 6 months of neoadjuvant androgen suppression, a size-reduction of the prostatic gland of 18% assessed by ultrasound and a serum PSA decrease from initially 27 ng/ml to 2.6 were seen. At 9 months follow-up the prostate showed a further decrease of volume as shown by MRI to 32 ml (35% reduction) compared to the pre-treatment volume of 49.5 ml (A, B). Serum PSA was reduced to less than 0.2 ng/ml. The testosterone level was 1.0 mmol/l. Treatment related side effects were within the expected range of combined therapy (urge and flush symptoms). After 12 months, MRI showed substantial fibrosis of the gland and nearby structures, such as the seminal vesicles. No metastases were seen. Serum PSA was still low (<0.2 ng/ml). The patient reported moderate urgency but no voiding problems of clinical significance. The flare symptomatic had improved. Androgen deprivation was continued.
Discussion
SRCCs are characterised by cells with intracytoplasmatic vacuoles and peripheral displacement of the nuclei, giving them a so-called signet-ring appearance. By definition, SRCCs are low differentiated adenocarcinomas with a diffusely infiltrating growth pattern. The tumours are well known in organs with mucin-producing epithelium, but do also sometimes occur in non-mucinous organs as breast, thyroid and urinary bladder Citation[1]. Prostate SRCCs differ from SRCC of other organs in that the signet-ring cells mostly do not stain with routine mucine stains or CEA. Thus, the diagnosis must be confirmed by immunohistochemistry, proving positivity for PSA or PAP. The use of radiological imaging is somehow uncertain. T2-weighted MRI showed no focal presentation of tumour within the prostatic gland, but a generalised infiltration pattern. This signal pattern is uncommon in the prostate, but more commonly picked up in signet ring cell carcinoma of the gastrointestinal tract. At follow-up MRI showed a more fibrotic signal pattern, confirming the effect of irradiation and hormonal therapy.
Due to their diffusely infiltrating growth pattern and thus often advanced stage at diagnoses, SRCCs are tumours with poor prognosis.
Similar to the report by Yoshimura et al. we achieved local control and remission in our patient applying definitive irradiation combined with hormonal therapy Citation[2]. In another report including Stage III disease a multimodal approach comprising RT or prostatectomy with hormonal treatment accomplished clinical benefit in two of four patients Citation[3]. On the other hand, most Stage IV cases at diagnosis had a poor prognosis Citation[3], Citation[4]. Thus, it is doubtful that patients will get a long-lasting response to hormonal manipulation only Citation[5]. The clinical experience is also limited to only sporadic cases. However, we believe that awareness of this entity, early diagnosis and combined treatment of SRCC of the prostate could improve the prognosis.
In summary, the treatment of SRCC with conformal radical radiotherapy in combination with androgen deprivation seems to be feasible with acceptable treatment related morbidity for this unusual prostate cancer.
References
- Young RH, Srigley JR, Amin MB, Ulbright TM, Cubilla AL. Tumors of the prostate gland, seminal vesicles, male urethra, and penis. Atlas of tumor pathology. Third Series, Fascicle 28. Armed Forces Institute of Pathology, Washington, DC, 2000.
- Yoshimura K, Fukui I, Ishikawa Y, et al. Locally-confined signet-ring cell carcinoma of the prostate: A case report of a long-term survivor. Int J Urol 1996; 3: 406–7
- Kazutoshi F, Hideki S, Takayasu G, Satoshi Y, Yasuhiro I. Primary signet ring cell carcinoma of the prostate: Report and review of 42 cases. Int J Urol 2004; 11: 178–81
- Keigo A, Hitoshi T, Shuji K, Shigeki O, Hisao N, et al. Signet-ring cell carcinoma of the prostate effectively treated with maximal androgen blockade. Int J Urol 2003; 10: 456–8
- Akagashi K, Hitoshi T, Kato S, et al. Signet-ring cell carcinoma of the prostate effectively treated with maximal androgen blockade. Int J Urol 2003; 10: 456–8