Abstract
Background. This study evaluated the accuracy of magnetic resonance imaging (MRI) for estimating residual tumor size after neoadjuvant chemotherapy in patients with locally advanced breast cancer and assessed whether the tumor pattern on MRI after chemotherapy influenced the accuracy of the MRI measurement of the residual tumor size. Patients and methods. Fifty patients who received neoadjuvant chemotherapy with doxorubicin and docetaxel for locally advanced breast cancer were evaluated with MRI before and after chemotherapy. We compared the residual tumor size measured by MRI with the pathologically determined size and investigated the influence of the residual tumor pattern on MRI (shrinkage, nest or rim, and mixed) and pathologic characteristics on the accuracy of the MRI measurement. Results. The correlation coefficient between the residual tumor sizes determined by MRI and by pathology was 0.645. The MRI measurement agreed with the pathologically determined size in 36 patients (72%) and disagreed in 14 patients (28%), overestimating the size in 13 (26%) and underestimating the size in one (2%). Disagreement appeared to be more frequent in the cases showing a nest or rim pattern than in those exhibiting a shrinkage pattern, although this was not statistically significant (p=0.119). Conclusions. MRI is an accurate method for predicting the extent of residual tumor after neoadjuvant chemotherapy; however, it may overestimate the residual disease, especially in cases showing a nest or rim tumor pattern and in those having combined lesions with ductal carcinoma in situ or multiple scattered nodules after neoadjuvant chemotherapy.
Neoadjuvant chemotherapy has become the standard treatment for patients with locally advanced breast cancer. The response of a tumor to chemotherapy affects disease-free survival Citation[1], Citation[2], and the extent of residual disease affects surgical planning. Thus, there is a great need for methods that accurately evaluate both the tumor response to chemotherapy and the extent of residual disease. Breast magnetic resonance imaging (MRI) is considered the most reliable method for monitoring tumor response to chemotherapy and for predicting the extent of residual disease after neoadjuvant chemotherapy Citation[3–5], although the reported accuracy of MRI for evaluating the extent of residual disease has differed according to the study group Citation[4], Citation[6–8]. In one study, the categorization of the initial MRI phenotype predicted the response to therapy Citation[9]. However, the relationship between the tumor pattern in response to chemotherapy and the accuracy of the MRI measurement of residual tumor has not been established. A better understanding of the relationship between the tumor response pattern and the MRI measurement would enable better surgical planning and more careful selection of candidates for breast conserving surgery.
We investigated the accuracy of MRI for predicting the extent of residual tumor in patients with locally advanced breast cancer who has received neoadjuvant chemotherapy with combined docetaxel and doxorubicin. We also analyzed the tumor patterns on MRI in response to chemotherapy and the characteristic pathologic findings to determine their influence on the accuracy of MRI measurements of residual tumor size.
Patients and methods
Patients and treatment
From July 2001 through September 2004, 61 women with locally advanced breast cancer at clinical stages IIB to IIIC, according to the American Joint Committee on Cancer, were prospectively enrolled in a study on neoadjuvant chemotherapy. All patients provided informed consent in compliance with the institutional review board. All patients were treated by neoadjuvant chemotherapy with doxorubicin 50 mg/m2 and docetaxel 75 mg/m2 i.v. every 3 weeks for three cycles, followed by lumpectomy or mastectomy as indicated. After surgery, all patients had undergone adjuvant treatment, including additional chemotherapy and/or radiation therapy as indicated. Of the 61 patients enrolled in the neoadjuvant chemotherapy trial with docetaxel and doxorubicin, 11 patients were excluded from our analysis for the following reasons: nine patients did not undergo pre- or post chemotherapy MRI and two patients didn't receive surgery. Finally, 50 patients were evaluated in the present study.
MRI technique
The 50 patients had undergone a physical examination and MRI just before and after three cycles of neoadjuvant chemotherapy. Breast MRI was performed with a three-phase dynamic technique that included one pre-contrast and two post-contrast scans after the injection of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ, USA) and a fat-suppressed, axial, 3D gradient-echo sequence on a 1.5 T MRI system (Signa CV/I, GE Medical Systems, Milwaukee, WI, USA) Citation[10]. The scan time for each dynamic scan was 3–4 minutes and the signal intensity of the first post-contrast scan reflected the signal intensity at a time of 1.5–2 minutes. After the entire dynamic scan, which lasted 6–8 minutes after the contrast injection, we obtained sagittal, axial, fat-suppressed and T1-weighted spin-echo images. All of the patients were imaged in the prone position using a dedicated breast coil. To improve visualization of the tumor area, two standard subtractions of pre-contrast images from the early post-contrast scan (SS1) and the late post-contrast scan (SS2) were performed. To analyze the kinetic pattern, a reverse subtraction (RS) of the late post-contrast scan from the early post-contrast scan was performed, and white areas on the RS image indicated washout -positive lesions Citation[10].
MRI measurement of tumor size and assessment of tumor response
On pre-chemotherapy MRI, we defined a tumor as an intensely enhancing mass with an irregular shape or margin on SS1. On post-chemotherapy MRI, we defined a tumor as an above-normal enhancing area at the previous tumor site on SS1 or SS2, considering that tumor enhancement is reduced after chemotherapy regardless of the presence of washout kinetics, as suggested in previous reports Citation[11–13]. The MRI interpretations and measurements were performed by the same radiologist who has five years experience in the interpretation of breast MRI. All measurements were made bi-dimensionally. For multiple tumors, the size of the largest index tumor was measured.
Tumor response was assessed according to the World Health Organization (WHO) criteria: complete response (CR, total disappearance of invasive cancer), partial response (PR, a reduction of ≥50% of the product of the two largest perpendicular dimensions), stable disease (SD, a reduction of < 50% or an increase of ≤25%), and progressive disease (PD, an increase of >25%). To assess tumor response, we expressed the tumor size as the product of the two largest perpendicular diameters.
The measurement of the residual tumor by MRI after neoadjuvant chemotherapy was compared with the pathologically determined measurement and categorized as in agreement, underestimated, or overestimated. We defined the tumor sizes measured by MRI and pathology as in agreement when the greatest tumor dimension measured by MRI was within 50–150% of the measurement determined by microscopic pathology Citation[14]. The MRI assessment was categorized as an underestimation when the longest diameter on imaging was < 50% that at pathology and as an overestimation when the longest diameter on imaging was > 150% that at pathology.
Analysis of tumor patterns on MRI and pathologic findings in cases showing disagreement between tumor size measurements after chemotherapy
The tumor patterns on MRI after neoadjuvant chemotherapy were classified as shrinkage, nest or rim, and mixed. When the shape of the tumor on MRI after chemotherapy was a shrunken single mass without division, the pattern was classified as a shrinkage pattern. For tumors that had divided into multiple masses or in which the central portion was replaced with a non-enhancing area to create a rim enhancement on post-chemotherapy MRI, the pattern was defined as a nest or rim pattern. A combination of these two patterns was defined as a mixed pattern. For cases showing disagreement between the measurements determined by MRI and pathology, we analyzed the pathologic findings and tumor patterns on MRI after neoadjuvant chemotherapy.
Statistical analysis
For the correlation between residual tumor size on MRI or palpation and that determined by pathology, Pearson's correlation analysis was used. The influence of the tumor pattern after chemotherapy was analyzed by the χ2 test through a cross validation procedure. P-values less than 0.05 were considered significant.
Results
Patient characteristics
Before chemotherapy, the primary tumors ranged from 2 to 14 cm (median, 7 cm) at the longest diameter as measured by palpation. Twenty-eight tumors (56%) were hormone (estrogen and/or progesterone) receptor positive, and 22 tumors (44%) were hormone receptor negative. The histological types were invasive ductal carcinoma (IDC) in 47 patients (94%) and invasive lobular carcinoma (ILC) in three patients (6%). All patients completed three cycles of neoadjuvant combination chemotherapy with doxorubicin and docetaxel. Thirty-six patients (72%) received modified radical mastectomy, and 14 (28%) received breast-conserving surgery. The patient characteristics are summarized in .
Table I. Patient characteristics before treatment.
Tumor response assessment
presents the average tumor size, expressed as the product of the two largest perpendicular diameters, before and after chemotherapy. The clinical overall response rate determined by palpation was 96%, and that determined by MRI was 68%. Pathologic CR was observed in three cases (6%), in one of these, the MRI examination correctly predicted the tumor response as CR ().
Figure 1. MRI before (A) and after (B) chemotherapy in a case accurately predicting residual disease. The tumor mass, measuring 1.7×2.0 cm before chemotherapy (A), disappeared completely on MRI after chemotherapy (B).
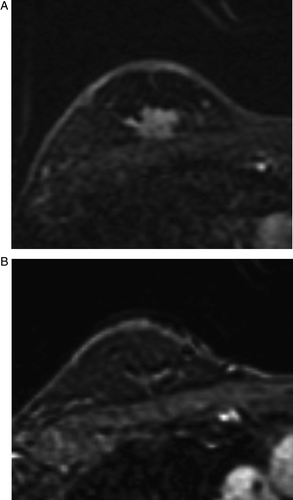
Table II. Tumor size expressed as the product of the two largest perpendicular diameters.
Assessment of residual tumor size after neoadjuvant chemotherapy
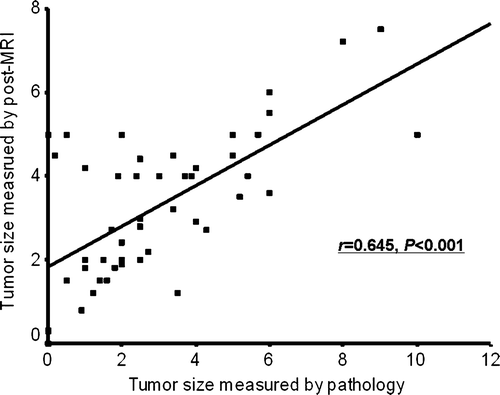
The correlation coefficient between the tumor size measured by MRI and that determined by pathology was 0.645 (p < 0.001, ). The MRI measurements agreed with the pathologic measurements in 36 patients (72%) and disagreed in 14 (28%), overestimating the tumor size in 13 patients (26%), and underestimating the size in one patient (2%).
Analysis of tumor patterns on MRI and pathologic findings in cases showing disagreement between tumor size measurements after chemotherapy
In six patients (12%), there was no significant change in the MRI findings following neoadjuvant chemotherapy. Of the remaining 44 patients, 20 (40%) exhibited a shrinkage pattern (), 20 (40%) had a nest () or rim pattern (), and four (8%) displayed a mixed pattern. Among the 20 patients with a shrinkage pattern, 16 (80.0%) showed agreement between the sizes measured by MRI and by pathology, whereas four (20%) showed disagreement. Among the 20 patients showing a nest or rim pattern, 11 patients (55.0%) showed agreement between the sizes measured by MRI and by pathology, and nine (45.0%) showed disagreement (). Disagreement was more frequent in the cases showing a nest or rim pattern than in those showing a shrinkage pattern, although this was not statistically significant (p = 0.119). summarizes the tumor patterns on MRI and pathologic findings after chemotherapy in the cases overestimated or underestimated by MRI.
Figure 3. MRI before (A) and after (B) chemotherapy in a case showing a shrinkage pattern of the tumor response. The mass, measuring as 5.0×3.2 cm before chemotherapy (A), decreased to 1.5×1.5 cm and showed a shrinkage pattern of MRI after chemotherapy (B). The size of the residual tumor according to the pathologic assessment agreed with that determined by MRI, 1.4 cm maximum.
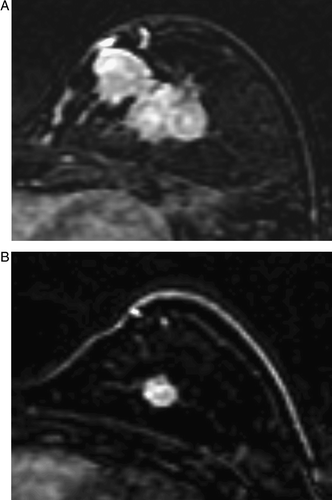
Figure 4. MRI before (A) and after (B) chemotherapy in a case showing nest pattern of the tumor response. The mass, measuring as 5.3×5.0 cm before chemotherapy (A), decreased slightly to 4.0×3.7 cm and showed a nest pattern on MRI after chemotherapy (B). A single inclusive measurement was given owing to multifocal enhancement after chemotherapy. The pathologic finding was a 1.8 cm, invasive ductal cancer with extensive intraductal components in a diffuse, unmeasurable, necrotic grayish area. MRI overestimated the size of the residual invasive tumor because it could not differentiate invasive from intraductal cancers
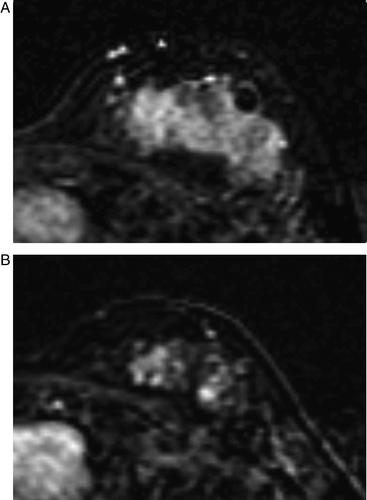
Figure 5. MRI before (A) and after (B) chemotherapy in a case showing a rim pattern of the tumor response. The mass, measuring as 1.8×2.0 cm before chemotherapy (A), decreased to 1.0×1.2 cm and showed a rim pattern on MRI after chemotherapy (B). The pathologic finding was IDC and measured 3.5 cm.
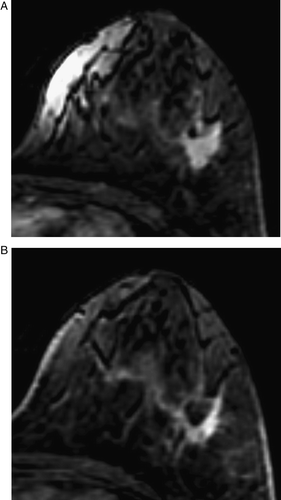
Table III. Agreement between the tumor sizes by MRI and by pathology, according to the tumor pattern.
Table IV. Tumor patterns on MRI and the pathologic findings in the 14 over or underestimated cases.
The pathologic findings in the overestimated cases included combined IDC and ductal carcinoma in situ (DCIS) (five cases), multiple scattered lesions (three cases), fibrosis and necrosis surrounding the tumor (two cases), lobular carcinoma in situ (LCIS) (one case), and multiple tumor emboli without mass formation (one case). In the one underestimated case, ILC was detected.
Discussion
Neoadjuvant chemotherapy followed by surgery has been shown to be effective for locally advanced breast cancer. This treatment combination is advantageous because it can provide evidence of in vivo responsiveness to the chosen chemotherapeutic agent Citation[15]. In addition, an inoperable advanced tumor may become operable, a large tumor may become amenable to breast-conserving surgery Citation[16], Citation[17], or micrometastatic lesions may be eradicated Citation[18] with this treatment. The findings of the National Surgical Adjuvant Breast and Bowel Project B-18 and other studies have demonstrated that the clinical or pathologic response of a primary tumor to chemotherapy is associated with both disease-free and overall survival Citation[1]. Therefore, the accurate assessment of both the response to neoadjuvant chemotherapy and the extent of residual disease may be crucial to establishing further therapeutic plans in patients with locally advanced breast cancer who have received neoadjuvant chemotherapy. MRI is considered more reliable than other conventional methods of breast cancer imaging such as mammography or breast sonography, for predicting the response to chemotherapy and the extent of residual disease after neoadjuvant chemotherapy Citation[3–5]. MRI has the advantages of providing a three-dimensional image of the breast, with high sensitivity in dense breast tissue, and using non-ionizing radiation. However, a few studies have questioned the accuracy of MRI on the grounds that contrast enhancement is affected by the cytotoxic and anti-angiogenic effects of chemotherapeutic drugs such as docetaxel, which might give a misleading over- or underestimation of residual tumor size Citation[7], Citation[11], Citation[19], Citation[20]. Measurement errors may result from a flawed observation by an examiner or from the failure to select the MRI or pathologic plane appropriate for revealing the largest tumor diameter, especially in tumors with star-like projectile margins in which it is difficult to differentiate between the tumor margin and normal parenchyma. Partridge et al. Citation[5] reported that the variation in tumor size measurements obtained on MR images was 11% between two independent observers.
We evaluated the performance of MRI for monitoring tumor size in patients with locally advanced breast cancer after receiving neoadjuvant chemotherapy with combined docetaxel and doxorubicin. In regards to determining the accuracy of imaging, there is no gold standard. Esserman et al. Citation[14] used a wider range for lesion extent concordance in evaluating their breast cancer patients. Usually the microscopic tumor margins extend beyond the gross borders. Therefore, we used generous criteria to identify the significant difference in the concordance. Although MRI accurately detected residual disease in 72% of the patients, there were some disagreements between the MRI and pathologic measurements, with tumor size being overestimated more often than underestimated. These discrepancies could have an impact on clinical decisions concerning further therapeutic approaches. One major factor contributing to the discrepancies may be the morphological patterns of the tumors in response to chemotherapy. The accuracy of MRI was limited in cases with a nest or rim pattern, which represent about half of the patients, in comparison with a shrinkage pattern, although this difference was not statistically significant. However, MRI has the potential to provide a quantitative measurement of both diameter and volume changes in response to chemotherapy. Therefore other considerations, including an assessment of peak percentage enhancement, might be useful. A three-dimensional volumetric assay by MRI may enhance the accuracy of tumor assessment.
There are several other factors that might have lead to the disagreement between the MRI and pathologic measurements. First, we observed a relatively high incidence of combined IDC and DCIS in post-chemotherapy pathologic specimens. Previous studies have shown a widely variable (10–90%) sensitivity in the detection of DCIS on MRI Citation[21], Citation[22], and these tumors have been identified as having variable morphology Citation[23], Citation[24]. This wide range of sensitivity and irregular morphology may contribute to a vague distinction between DCIS and invasive cancer on MRI. DCIS and invasive cancer were not differentiated on MRI and both were measured as a single value, however, only the invasive tumor was regarded as residual disease in the pathologic TNM staging. Therefore, in tumors combined with DCIS, an overestimation is inevitable by MRI. However, in view of surgical planning, and not in the aspect of tumor response, this type of overestimation doesn't matter because the DCIS as well as the invasive tumor should be removed. Accordingly, it is not relevant to include this among the overestimations. Second, three overestimated cases had multiple adjacent tumor nodules. In those cases, while the radiologist measured the area of multiple nodules as a single value on MRI, the pathologist measured only the largest tumor or described it as multiple individual values. Although the radiologist tried to measure the area of multiple nodules as individual ones, it was difficult when the multiple tumors were too closely aggregated. Third, residual enhancement from reactive inflammation, which is caused by the tumor response and the healing process, may contribute to overestimation by MRI Citation[23]. It has been reported that a change in tumor vascularity in response to chemotherapy may decrease or delay enhancement in tumors after treatment Citation[11–13], which can yield false-negative results. Therefore, we regarded any enhancement above normal at the original tumor site to be residual tumor. Measurements made at the delayed phase may result in an overestimation by including non-specific enhancement from chemotherapy-induced tumor regression. In our study, two cases of pathologic CR showed residual enhancement at the original tumor site that was regarded as residual tumor, however, one case was attributable to the presence of a micro-focus of DCIS, and the other was attributable to reactive inflammation only. Fourth, a taxane-induced increase in vascular permeability and capillary protein leakage can lead to augmented gadolinium uptake by the tumor Citation[25], which would produce an overestimation.
A potential advantage of our study is the homogeneity of the clinical, radiological, and pathologic assessments. All patients were prospectively treated with neoadjuvant docetaxel and doxorubicin, allowing us to assess the taxane- induced vascular permeability or anti-angiogenic effect of docetaxel on breast MRI.
Conclusion
MRI is an accurate assessment tool for predicting residual tumor after chemotherapy; however, it can overestimate the residual invasive disease, especially in cases showing a nest or rim pattern in response to chemotherapy and in cases with combined lesions with DCIS or multiple scattered nodules. The response patterns to chemotherapy on MRI and variable pathologic characteristics may affect the discrepancies between pathologic and MRI assessments. Cautious interpretation is needed in determining the extent residual disease and in planning surgery.
References
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001; 30: 96–102
- Bear HD, Anderson S, Smith RE, Geyer CE, Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006; 24: 2019–27
- Warren RM, Bobrow LG, Earl HM, Britton PD, Gopalan D, Purushotham AD, et al. Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy?. Br J Cancer 2004; 90: 1349–60
- Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. Am J Roentgenol 2003; 181: 1275–82
- Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. Am J Roentgenol 2002; 179: 1193–9
- Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kuhn T. Breast MRI for monitoring response of primary breast cancer to neoadjuvant chemotherapy. Eur Radiol 2002; 12: 1711–9
- Denis F, Desbiez-Bourcier AV, Chapiron C, Arbion F, Body G, Brunereau L. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur J Surg Oncol 2004; 30: 1069–76
- Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs histologic measurement of breast cancer following chemotherapy; comparison with x-ray mammography and palpation. J Magn Reson Imaging 2001; 13: 868–75
- Esserman L, Kaplan E, Partridge S, Tripathy D, Rugo H, Park J, et al. MRI phenotype is associated with response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol 2001; 8: 549–59
- Choi N, Han BK, Choe YH, Kim HS. Three-phase dynamic breast magnetic resonance imaging with two-way subtraction. J Comput Assist Tomogr 2005; 29: 834–41
- Wasser K, Sinn HP, Fink C, Klein SK, Junkermann H, Ludemann HP, et al. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur Radiol 2003; 13: 1213–23
- Rieber A, Zeitler H, Rosenthal H, Gorich J, Kreienberg R, Brambs HJ, et al. MRI of breast cancer: Influence of chemotherapy on sensitivity. Br J Radiol 1997; 70: 452–8
- Tsuboi N, Ogawa Y, Inomata T, Yoshida D, Yoshida S, Moriki T, et al. Changes in the findings of dynamic MRI by preoperative CAF chemotherapy for patients with breast cancer of stage II and III: Pathologic correlation. Oncol Rep 1999; 6: 727–32
- Esserman L, Hylton N, Yassa L, Barclay J, Frankel S, Sickles E. Utility of magnetic resonance imaging in the management of breast cancer: Evidence for improved preoperative staging. J Clin Oncol 1999; 17: 110–9
- Chollet P, Amat S, Cure H, de Latour M, Le Bouedec G, Mouret-Reynier MA, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer 2002; 86: 1041–6
- Deo SV, Bhutani M, Shukla NK, Raina V, Rath GK, Purkayasth J. Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). J Surg Oncol 2003; 84: 192–7
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15: 2483–93
- Kim R, Osaki A, Toge T. Current and future roles of neoadjuvant chemotherapy in operable breast cancer. Clin Breast Cancer 2005; 6: 223–32
- Yeh E, Slanetz P, Kopans DB, Rafferty E, Georgian-Smith D, Moy L, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. Am J Roentgenol 2005; 184: 868–77
- Bhujwalla ZM, Artemov D, Natarajan K, Solaiyappan M, Kollars P, Kristjansen PE. Reduction of vascular and permeable regions in solid tumors detected by macromolecular contrast magnetic resonance imaging after treatment with antiangiogenic agent TNP-470. Clin Cancer Res 2003; 9: 355–62
- Neubauer H, Li M, Kuehne-Heid R, Schneider A, Kaiser WA. High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: Characteristic findings for signal increase and morphological pattern of enhancement. Br J Radiol 2003; 76: 3–12
- Viehweg P, Lampe D, Buchmann J, Heywang-Kobrunner SH. In situ and minimally invasive breast cancer: Morphologic and kinetic features on contrast- enhanced MR imaging. MAGMA 2000; 11: 129–37
- Liberman L, Morris EA, Lee MJ, Kaplan JB, LaTrenta LR, Menell JH, et al. Breast lesions detected on MR imaging: Features and positive predictive value. Am J Roentgenol 2002; 179: 171–8
- Hylton N. Magnetic resonance imaging of the breast: Opportunities to improve breast cancer management. J Clin Oncol 2005; 10: 1678–84
- Delille J-P, Slanetz PJ, Yeh ED, Halpern EF, Kopans DB, Garrido L. Invasive ductal breast carcinoma response to neoadjuvant chemotherapy: Noninvasive monitoring with functional MR imaging pilot study. Radiology 2003; 228: 63–9