Abstract
A retrospective analysis of the relation between the presence of casting-type calcifications on the mammogram and the prognosis of breast cancer was performed. The mammographic tumor features and other characteristics (invasive tumor size, histological tumor type, grade, nodal, hormone receptor and HER2 status, presence of lymphovascular invasion) of 55 high-risk breast cancers were studied. After a median follow-up time of 29.1 months, the median relapse-free survival and overall survival times among breast cancer patients with tumors associated with casting calcifications were 26.6 and 29.6 months, respectively. The corresponding parameters among patients with tumors not accompanied by casting calcifications were 54.4 and >58.5 months, respectively. Significant associations were found between the presence of casting calcifications and the risks of relapse (HR = 3.048, 95% CI: 1.116–8.323, p = 0.030) or death (HR = 3.504, 95% CI: 1.074–11.427, p = 0.038). Positive associations were found between casting calcifications and ER/PR negativity (p = 0.015 and p = 0.003, respectively) and HER2 overexpression (p = 0.019). Our findings support the theory that breast tumors associated with casting-type calcifications at mammography comprise a disease entity which exhibits significantly more aggressive behavior and a poorer outcome than do cancers with other mammographic tumor features.
Breast cancer is a heterogeneous disease as regards its gene-expression profile Citation[1], Citation[2], its pathological and its mammographic appearance, its biological behavior Citation[3–7] and the response to oncological treatment Citation[1], Citation[8]. The major progress achieved in the past decade in the adjuvant treatment of early breast cancer has contributed to an improvement in breast cancer-related survival Citation[9]. Improved characterization of the different cancers may promote a better estimation of the outcome of the disease and the potential benefit provided by the various forms of adjuvant therapy Citation[1], Citation[8]. The prognostic indicators reflect the stage of the cancer reached during the progression process and the degree of its aggressiveness. The tumor size and nodal status reflecting the stage of the cancer are classical prognostic factors. The histopathological type, the grade, the presence or absence of lymphovascular invasion (LVI), the expression of the hormone receptors and HER2 rather relate to the biological behavior of the cancer Citation[4], Citation[10]. Nonetheless, more specific indicators for the better identification of high-risk cases are needed. The mammographic appearance has recently been suggested as an independent prognostic factor for mammography screening-detected breast cancers Citation[4–7], Citation[11]. Among cancers measuring <15 mm detected by mammography screening, the presence of casting-type calcifications has been demonstrated to be a prognostic factor which carries a significantly (9-fold) higher risk of death as compared with cancers not associated with this mammographic abnormality Citation[4]. We set out to study whether this mammographic feature might be indicative of a different prognosis in more advanced operable breast cancer cases. Accordingly, we performed a retrospective analysis of the mammographic appearance of 55 high-risk breast cancers of patients receiving uniform oncological treatment. Additionally, we studied the relation between the presence of casting-type calcifications and the other characteristics of the tumors.
Material and methods
The tumors and clinical outcomes of 55 consecutive high-risk breast cancer patients enrolled in an adjuvant chemotherapy clinical study after surgery Citation[12] were studied. Eligible patients had operable breast cancer with either ≥4 lymph node metastases, or a > pT1 grade-III, ER and PR-negative tumor with lymph node metastasis, or a grade-III, ER and PR-negative tumor of any size with lymph node metastasis and vessel invasion, or a T > 3 cm, grade-III, ER and PR-negative tumor. The primary tumor had to have been resected with a clear margin of at least 5 mm, but of at least 10 mm provided an extensive ductal component was present. The presence of distant metastases had to be excluded by means of physical examination, chest x-ray, abdominal ultrasound and bone scan. The study had been approved by the Institutional Review Board of the University of Szeged, and all enrolled patients gave written informed consent before being registered as participating in the study. Forty-seven of the 55 patients enrolled completed 24-week adjuvant dose-dense sequential doxorubicin-paclitaxel-cyclophosphamide chemotherapy, while in eight cases the chemotherapy was terminated earlier. After the chemotherapy, all of the patients underwent postoperative locoregional radiotherapy unless having had a relapse earlier, and those with hormone receptor-positive tumors received tamoxifen Citation[12].
The original mammograms taken before surgery were retrospectively reviewed. All mammograms were classified by a single radiologist (KO) blinded to the pathological and clinical data. The lesions were categorized by their radiomorphological appearance into two groups: the lesions with casting calcifications (BI-RADS: fine linear branching calcifications), including any associated abnormality, and tumors in which casting-type calcifications were absent Citation[3], Citation[4].
After a median follow-up time of 29.1 months, the relapse-free survival (RFS) and overall survival (OS) were studied in relation to the mammographic features, the age of the patient and the characteristics of the tumor (the tumor size, the number of involved lymph nodes, the histology, the grade, the hormone receptor status, the HER2 status, and the presence of LVI). RFS was defined as the time from enrolment to any disease recurrence (local, regional, distant relapse or a contralateral breast cancer). OS was defined as the time from enrolment to death.
We wished to learn whether there were significant initial differences between the risks of relapse or death among the patients as a function of the radiomorphological appearance of the tumors. Hence, the 10-year risks of relapse and mortality were calculated on the basis of the conventional prognostic factors (the age, the ER status, grade, size and nodal status of the tumor) using the software Adjuvant! Citation[13]. This system provides predicted outcomes based on the patient and tumor characteristics. Calculations were made both with and without the inclusion of the HER2 status. Estimated risks of relapse and mortality were compared between the cases with and those without casting calcifications.
Statistical analyses
For the categorical parameters, χ2 or Fisher tests were applied; for the analysis of continuous data, the Mann-Whitney U-test was used. RFS and OS times were calculated by means of the Kaplan-Meier method. Comparisons relating to the presence of casting calcifications were made with the log-rank test. To estimate the effect of casting-type calcifications and the conventional prognostic factors on long-term disease-outcome, the Cox proportional hazards model was used. A stepwise selection method was performed using the likelihood-ratio statistics based on the maximum partial likelihood estimates.
Results
The mean patient age was 51.7 (33–70) years; 49% had ER/PR-negative and 20% HER2- + +/ + ++ tumors; the median number of positive lymph nodes was 6, and 1/3 of the patients had ≥10 involved lymph nodes. Sixty per cent of the tumors were pT2, and 18% were pT1. Most of the breast tumors were grade 3 invasive ductal cancers, and 2/3 exhibited LVI. Among the 55 high-risk breast cancers, ten displayed the characteristics of casting-type calcifications. Patient- and tumor-related data according to the mammographic tumor features are presented in . Significantly more tumors associated with casting calcifications were of grade 3 (p = 0.036), HER2-positive (p = 0.012), ER-negative (p = 0.015) or PR-negative (p = 0.003) than in the other group. No other characteristics of the tumors were related to the mammographic appearance ().
Table I. Patient- and tumor-related characteristics of 55 high-risk breast cancer cases displaying the presence or absence of casting-type calcifications on the mammogram.
In order to determine whether there was an ab ovo difference in the prognosis of the study patients, depending on the presence or absence of casting-type calcifications, we performed an analysis based on the conventional prognostic factors. The risks of relapse and death calculated by means of the software Adjuvant! are shown in . There was a trend toward a higher mortality risk in the group with casting-type calcifications as compared to the group without them, which became statistically significant when the HER2 status was included ().
Table II. Risks of relapse and death based on conventional prognostic factors (age, grade, ER status, tumor size, nodal status with or without HER2 status) among the tumor groups with or without casting-type calcifications, as indicated by the software Adjuvant!
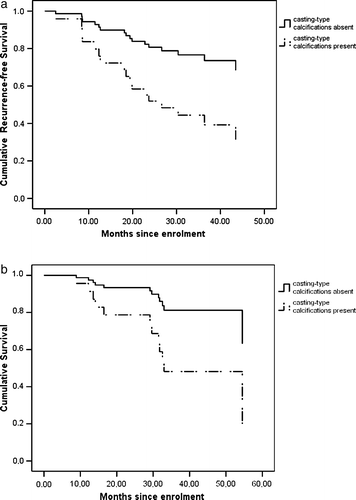
After a median follow-up time of 29.1 months, seven (70%) of the ten patients with casting calcifications had relapsed, and five (50%) had died. Among these patients, five had distant metastases, one had a local relapse, and one developed a second primary breast cancer. The latter two patients underwent surgery and adjuvant postoperative treatment, and were alive without evidence of disease at the end of the follow-up period. Eleven (24.4%) of those patients who had no casting-type calcifications on the mammogram relapsed, and seven (15.6%) died. Thus, 42% of all death events occurred in the group of patients having had tumors exhibiting casting calcifications. RFS and OS were 26.6 and 29.6 months in the group with casting-type calcifications, and 54.4 and >58.8 months (median), respectively, in the other group (p = 0.022 and 0.028, respectively; log-rank test). In order to estimate long-term prognosis depending on the presence of casting-type calcifications, the tumor grade, the LVI, the expressions of ER/PR and HER2, the tumor size and the nodal status were studied in a Cox proportional hazards model. shows cumulative relapse-free survival and overall survival associated with the presence or absence of casting calcifications on the mammogram. In the presence of casting-type calcifications, the risks of relapse and death were increased, with HR = 3.048 (95% CI: 1.116–8.323, p = 0.030) and HR = 3.504 (95% CI: 1.074–11.427, p = 0.038). No other patient- or tumor-related factor exerted a significant effect on the risk of relapse.
Discussion
This retrospective analysis was performed to assess the value of casting-type calcifications as a mammographic indicator of a poor prognosis in high-risk breast cancer patients. In agreement with earlier studies Citation[3–7], Citation[11], we found the presence of casting-type calcifications to be the most powerful independent prognostic factor in a group of high-risk operable breast cancer cases. Our results contribute to and support the earlier finding that breast tumors associated with casting-type calcifications on the mammogram comprise a disease entity which is clearly different from cancers with other mammographic tumor features.
The previous studies on the prognostic role of casting-type calcifications were performed on screen-detected small cancers Citation[3–7], Citation[11]. Our study group consisted of more advanced, but operable cases with large and usually node-positive tumors. We found more than 3-times higher risks of relapse and death in the group of tumors associated with casting-type calcifications as compared with the group without them. Tabár et al. reported that the risk of death was 32-times higher in screening-detected invasive breast cancers smaller than 10 mm and associated with casting-type calcifications as compared with those without such calcifications. The difference in the risk of fatality was smaller between the cases with and those without casting-type calcifications when the tumors measured 10–15 mm Citation[5]. We were interested in whether the worse prognosis associated with casting-type calcifications would still exist in a set of high-risk, mostly clinically detected breast cancers, and it emerged that their presence was paralleled by a significantly poorer prognosis.
We analyzed whether the conventional prognostic factors differed between the group of breast tumors associated with casting-type calcifications and the group which did not display this mammographic feature. The tumor size and the nodal status reflecting primarily the stage of the disease did not reveal any difference between the two groups. The grade 3 phenotype was significantly more common among the tumors associated with casting-type calcifications, which is in accordance with the data of other authors Citation[11], Citation[14].
Zunzunegui et al. studied a group of 12 breast cancer patients with invasive tumors who exhibited casting-type calcifications Citation[7]. These tumors were smaller than the ones in our study, and all contained an extensive intraductal component. Half of them were ER/PR-negative, while 60% were HER2-positive. Our data are in consistence with those results, since all the tumors associated with casting-type calcifications in our study contained extensive high grade DCIS components. In fact, we found an even higher proportion of hormone receptor-negative cancers among those with casting-type calcifications. Moreover, HER2 positivity was typical in the cancer cases with casting-type calcifications. Our findings also accord with those of Millis et al., who demonstrated that the histological type of the DCIS component in invasive tumors was highly correlated with the grade of the infiltrative part Citation[15], and predicted survival in 215 infiltrating ductal carcinomas after a median follow-up time of 11.2 years Citation[16].
In the study of MacMillan et al., residual microscopic disease after breast conservation surgery was associated with the mammographic appearance of casting calcifications and predicted local recurrence Citation[17]. Casting-type calcifications were the only independent mammographic feature that predicted residual cancer cells in the tumor bed and the consequent risk of local relapse Citation[18]. Among our seven relapsed patients with cancers associated with casting-type calcifications, one had a local relapse on the chest wall.
With a view of analyzing whether the use of conventional prognostic factors could have predicted a significant difference between the groups of tumors with or without casting-type calcifications, we compared the risks of relapse and mortality as predicted by the software Adjuvant! Citation[13]. Although there was a non-significant trend toward a greater mortality risk in the cases exhibiting casting-type calcifications, there was no difference in the risk of relapse. The difference in predicted mortality risk between the two groups became statistically significant when the HER2 status was included. This latter result does not contradict, but rather strengthens the hypothesis that a positive HER2 status and casting-type calcifications are common features of the same tumor type with an extremely poor prognosis.
Tabár et al. proposed that invasive breast cancers associated with casting-type calcifications and grade 3 DCIS comprise a different tumor type in a more advanced stage than indicated by the conventional prognostic factors, compared with their counterparts without this mammographic feature Citation[3–5]. Neoductgenesis in the high-grade DCIS components, as a special process promoting vascular invasion, and consequently excessive lymphatic and hematogenous spread is suggested as a key underlying mechanism for the worse outcome in these tumors Citation[5]. In accordance with the findings of Tabar et al., despite the outstanding difference in outcome, we did not observe any difference between the tumors with or without casting-type calcifications, as concerns conventional prognostic factors (tumor size, number of positive lymph nodes, LVI) Citation[3], Citation[5]. Our study clearly demonstrated that tumors with casting calcifications on the mammogram are typically of ductal type, of grade 3, ER and PR negative and HER2 positive. Based on these results, we believe that tumors associated with casting-type calcifications display different biological behavior with higher rates of proliferation and progression than other breast cancers. It is also probable that tumors with casting-type calcifications are more resistant to chemotherapy than tumors without them. This is indicated by the poor survival of the patients with invasive cancers measuring 1–14 mm associated with casting-type calcifications, irrespective of the therapeutic regimen applied Citation[5]. Our data points to relative therapy resistance. Our study group consisted of high-risk, uniformly treated breast cancer patients. Those patients with casting-type calcifications relapsed and died soon after treatment, despite the administration of adjuvant dose-dense sequential paclitaxel-containing chemotherapy. There is an urgent need for the development of effective treatment methods for this special type of cancer, as none currently exists Citation[5]. Primary systemic treatment could be an appropriate setting in which to test the antitumor potential of new pharmaceuticals, including different cytostatics and molecularly targeted therapies. The two most plausible of the latter group of agents are possibly trastuzumab and bevacizumab. Our results demonstrate that the presence of casting-type calcifications is significantly associated with the overexpression of HER2. Five randomized studies recently provided overwhelming evidence that the addition of trastuzumab to the adjuvant chemotherapy may halve the risk of early relapse Citation[19–22]. The vascular invasion could be prevented and the pathologically leaky vasculature could be normalized by use of the anti-VEGF bevacizumab Citation[23]. Since most of the tumors associated with casting-type calcifications were ER/PR-negative, there is little likelihood of benefit from hormonal therapy in this group of patients.
We believe that the detection of casting-type calcifications at mammography should be a warning sign of aggressive tumor behavior, irrespective of the stage of the tumor. Although the number of patients in our analysis was rather modest, the striking difference in outcome appears to deserve attention. Our observations warrant further analyses, and stress the need for the testing of new treatment options for the high-risk group of breast cancers associated with casting-type calcifications.
Acknowledgements
This analysis was inspired by (but is otherwise independent of) the highly stimulating work of László Tabár (Falun Central Hospital, Sweden).
References
- Van de Vijver M. Gene-expression profiling and the future of adjuvant therapy. Oncologist 2005; 10: 30–4
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–74
- Tabar L, Chen H, Duffy SW, Yen MF, Chiang CF, Dean PB, et al. A novel method for prediction of long-term outcome of women with T1a, T1b and 10–14 mm invasive breast cancers: A prospective study. Lancet 2000; 355: 429–33
- Tabar L, Dean PB, Kaufman CS, Duffy SW, Chen HH. A new era in the diagnosis of breast cancer. Surg Oncol Clinics NA 2000; 9: 233–77
- Tabar L, Tony Chen HH, Amy Yen MF, Tot T, Tung TH, Chen LS, et al. Mammographic tumor features can predict long-term outcomes reliably in women with 1–14-mm invasive breast carcinoma. Cancer 2004; 101: 1745–59
- Thurfjell E, Thurfjell MG, Lindgren A. Mammographic finding as predictor of survival in 1–9 mm invasive breast cancers. Worse prognosis for cases presenting as calcifications alone. Breast Cancer Res Treat 2001; 67: 177–80
- Zunzunegui RG, Chung MA, Oruwari J, Golding D, Marchant DJ, Cady B. Casting-type calcifications with invasion and high-grade ductal carcinoma in situ. Arch Surg 2003; 138: 537–40
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. Multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351: 2817–26
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Webster LR, Bilous AM, Willis L, Byth K, Burgemeister FC, Salisbury EL, et al. Histopathologic indicators of breast cancer biology: Insights from population mammographic screening. Br J Cancer 2005; 92: 1366–71
- Peacock C, Given-Wilson RM, Duffy SW. Mammographic casting-type calcification associated with small screen-detected invasive breast cancers: Is this a reliable prognostic indicator?. Clin Radiol 2004; 59: 165–70
- Kahan Z, Uhercsak G, Hajnal-Papp R, Boda K, Thurzo L. Dose-dense sequential adriamycin-Paclitaxel-cyclophosphamide chemotherapy is well tolerated and safe in high-risk early breast cancer. Oncology 2005; 68: 446–53
- Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 2001; 19: 980–91
- James JJ, Evans AJ, Pinder SE, Macmillan RD, Wilson AR, Ellis IO. Is the presence of mammographic comedo calcification really a prognostic factor for small screen-detected invasive breast cancers?. Clin Radiol 2003; 58: 54–62
- Lampejo OT, Barnes DM, Smith P, Millis RR. Evaluation of infiltrating ductal carcinomas with a DCIS component: Correlation of the histologic type of the in situ component with grade of the infiltrating component. Semin Diagn Pathol 1994; 11: 215–22
- Millis RR, Ryder K, Fentiman IS. Ductal in situ component and prognosis in invasive mammary carcinoma. Breast Cancer Res Treat 2004; 84: 197–8
- MacMillan RD, Purushotham AD, Cordiner C, Dobson H, Mallon E, George WD. Predicting local recurrence by correlating pre-operative mammographic findings with pathological risk factors in patients with breast cancer. Br J Radiol 1995; 68: 445–9
- Malik HZ, Wilkinson L, George WD, Purushotham AD. Preoperative mammographic features predict clinicopathological risk factors for the development of local recurrence in breast cancer. Breast 2000; 9: 329–33
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–84
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–72
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Pawlicki M, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study [abstract 1]. Breast Cancer Res Treat 2005; 94(Suppl 1)S5
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006; 354: 809–20
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005; 10(Suppl 3)S20–S29