Abstract
Introduction: Alterations in eicosanoid metabolism is well established in a variety of malignant tumors, particularly colorectal carcinoma. Recent studies in our laboratory have emphasized a role for EP subtype receptors in progression of colorectal cancer and disease specific mortality. Therefore, the aim of the present study was to extend our knowledge to include additional receptor expression (DP1, DP2, FP, IP, TP) for prostanoids (PGD2, TXA2, PGF2α, PGI2) in relationship to tumor stage, differentiation and progression of colorectal cancer. Material and methods: Total RNA from 62 tumors and adjacent normal colon tissue (n = 48) was extracted. Quantification of receptor expression was performed by realtime PCR and related to the expression of an appropriate housekeeping gene (GAPDH). Tumors were assessed according to Dukes A-D (stage I-IV). Results: DP1, DP2, FP and IP receptor subtypes displayed significantly reduced overall expression in tumor tissue compared to normal colon tissue, while the TP receptor subtype showed significantly higher expression in tumor tissue. Overall expression of the prostanoid receptors in tumor tissue was not related to clinical indexes as tumor stage and tumor cell differentiation evaluated by multivariate analyses. Cultured colorectal cancer cell lines with low (HT-29) and high (HCA-7) intrinsic PGE2 production at confluent state did not express DP1 and IP receptor subtypes, but displayed low expression of DP2, FP and TP receptor subtypes. Conclusion: The results in the present study indicate imbalanced expression of prostanoid receptors in colorectal cancer compared to normal colon tissue without clear cut relationship to disease progression. Therefore, future studies should be performed on defined cells within the tumor tissue compartment determining whether any prostanoid receptor(s) is useful as a molecular target in treatment or prevention of colorectal cancer.
Eicosanoids are involved in cell proliferation, adhesion, migration, differentiation, apoptosis, tumor invasiveness and angiogenesis Citation[1–6]. Consequently, inhibition of cyclooxygenases (COX-1/COX-2) producing eicosanoids, by non-steroid anti-inflammatory drugs (NSAIDs), has confirmed to influence on tumor growth, progression and size-reduction of colon cancer Citation[7–10]. Eicosanoids act as autocrine or paracrine mediators through binding to G-protein coupled receptors in the cell membrane altering cellular levels of cAMP with changed concentrations of Ca2 + - ions, affecting different signaling pathways in the cell Citation[11].Thus, eicosanoids are closely related to the behavior of malignant tumors, where elevated levels of prostaglandin E2 (PGE2), PGD2 and tromboxane A2 (TXA2) as well as reduced levels of PGF2α and PGI2 (prostacyclin) have been reported of in tumor tissue Citation[12–14]. Normally, PGI2 mediates increased vascular permeability and promotes inflammation. Opposing PGI2 is TXA2, a strong constrictor of vasculature and a potent stimulator of platelets aggregation. PGF2α acts on smooth muscle cells whereas PGD2 promotes production of cytokines and chemokines during antigenic challenges Citation[15], which all may decide tumor behavior Citation[16]. Accordingly, recent work in our laboratory, where COX and PGE2 receptors (EP1–4) as well as PPARγ were analyzed in colorectal tumors, displayed that increased expression of EP2 receptor predicted poor survival following primary surgical operation Citation[17]. Therefore, the present study was aimed to map remaining eicosanoid receptor expression in human colorectal cancer and corresponding normal colon tissue in relationship to tumor characteristics as histopathology (grade) and tumor progression (stage).
Material and methods
Patients
Tumor and large bowel tissue samples were collected from 62 unselected patients at primary operation for colorectal carcinoma between 2001 to 2004 at Sahlgrenska University Hospital (n = 14) and a regional county hospital (Uddevalla, n = 48) of Sweden. The group consisted of 50% males and 50% females with a median age of 73 years (range 40 to 91 years) at surgery. Disease specific mortality was as expected accounting for age and tumor stage. Tumors were histologically classified as Dukes A (10), Dukes B (26), Dukes C (16) and Dukes D (10), corresponding to tumor stage I-IV respectively. All patients underwent surgery as the only curative treatment and none received neoadjuvant radio-chemotherapy according to individual patient judgments and local hospital routines.
Tumor tissue material
Tumor (n = 62) and normal large bowel tissue samples (n = 48) (down to the serosa layer) were collected during surgery and kept fresh frozen in liquid nitrogen and stored in −70°C until analysis. Certified pathologists staged all tumors. Tumor samples for RNA analysis contained around 70–80% tumor cells according to microscopic visual inspection.
Cell culture
Prostanoid receptor expression was also studied in two well-differentiated human colon adenocarcinoma cell lines with low (HT-29, ATCC) and high (HCA-7, Sigma-Aldrich, St.Lois, USA) intrinsic PGE2 production. HCA-7 cells were chosen since these cells are reported to express all EP1–4 subtype receptors while HT-29 cells lack expression of EP2 and EP3 as confirmed. Cultured cells were maintained in monolayers in flasks (25 cm2) from Corning in a humidified (95%) incubator at 37°C with 5% CO2. HT-29 cells were maintained in McCoy's 5A medium (ICN Biomedicals, Aurora, OH, USA) supplemented with 10% fetal calf serum (FCS). The split ratio was 1/12. HCA-7 cells were maintained in Dulbecco's modified Eagle's medium D6546 (DMEM) from Sigma-Aldrich with 10% FCS and a split ratio of 1/5. Penicillin, streptomycin and L-glutamine were added to the concentration of 100 U/ml, 100 µg/ml respectively 292 µg/ml (Bio Whittaker, Europe, Verviers, Belgium). Weekly medium changes (McCoy's 5A/DMEM + 2% FCS) were provided.
RNA extraction and cDNA synthesis
Total RNA from 48 tumors and adjacent normal colon tissue (29 patients) was extracted with GenElute™ Mammalian Total RNA Kit from Sigma. The remaining 14 tumors and adjacent tissue (14 patients) RNA was extracted with RNeasy® Fibrous Tissue Midi kit from Qiagen according to the protocol for Total RNA Isolation from Fibrous Tissue enclosed by the manufacturer. RNA from cultured cells (HT-29 and HCA-7) was extracted with RNeasy® Mini kit from Qiagen according to enclosed protocol. Purity and concentration of extracted RNA were checked and quantified by reading at 260 and 280 nm in a spectrophotometer (Pharmacia GeneQuant RNA/DNA Calculator). A quality control and concentration measurement of RNA was performed in the Agilent Technologies 2100 Bioanalyzer per manual for eukaryotic total RNA before cDNA synthesis. One µg of RNA was used in BD Advantage™ RT-for-PCR kit (BD Bioscience) according to the manufacturer's instruction. Sterile water substituted for RNA in negative controls. Samples without RT-polymerase were run to exclude genomic contamination.
Realtime PCR
Realtime PCR was performed in the LightCycler 1.5 with LightCycler FastStart DNA Master SYBR Green1 or FastStart DNA MasterPlus SYBR Green1 kit to analyze relative expression of the five prostanoid receptor genes in tumor tissue and normal colon tissue according to a standard protocol (Roche). Primers were added to a final concentration of 0.5 µM and 2/5 µl cDNA (SYBR Green I/PLUS) were added to each capillary (). All samples were performed in duplicate and related to the expression of an appropriate housekeeping gene (GAPDH), as confirmed in previous work Citation[17]. Reactions were optimized regarding MgCl2 concentration, annealing temperature and primer concentration. The products were checked in the Bioanalyzer 2100 (Agilent Technologies) according to the protocol for DNA1000 for correct amplicon size. PCR-graded water was used as negative control in every reaction. Results were produced by use of the relative standard curve method where the standard specimen was a colon tumor (Dukes C) resected at Sahlgrenska University Hospital. All samples were confirmed to be within the range of the standard curve. EP1–4 subtype receptor expression in cultured cells were measured as described elsewhere Citation[17]. Standard samples displayed expression as expected and negative control samples showed no expression.
Table I. The primers used in LightCycler® realtime PCR from 5′ to 3′.
Statistics
Results are presented as relative gene expressions per GAPDH expression and presented as mean±standard error of units obtained from the LightCycler® datafiles. The statistical testing was performed by non-parametric tests (Kruskal-Wallis and Mann-Whitney). Survival analysis was performed according to Kaplan-Meier and tested statistically with the log rank technique. Alive patients were censored in survival analyses. Multivariate regression analysis was performed according to standard procedures with disease specific mortality as dependent factor (Statview 5.0.1, SAS Institute Inc.). P < 0.05 was regarded statistically significant and p < 0.10 a trend to significance in two-sided tests.
Results
DP1 and IP receptor subtypes displayed clear cut significantly lower overall expression in tumor tissue compared to normal colon tissue, while the TP receptor subtype showed significantly higher expression in tumor tissue than in normal colon tissue, although a more differentiated pattern appeared when normal colon tissue was compared to Dukes A-D in a multigroup analysis (, ). Expression of any subtype prostanoid receptor was not related to tumor stage ( and ) or tumor cell differentiation in univariate analysis (). Multivariate regression analysis did not indicate any of the subtype receptors to be a significant predictor of survival (not shown), although disease specific survival in itself was related to tumor stage as expected (not shown). Both tumor tissue specimen and normal colon tissue were simultaneously obtained from 43 patients. Receptor expression analysis within patient group was performed to check the validity of our results from the cross-sectional analyses derived on the entire patient material (). Results of receptor expression within patient group agreed with cross-sectional results for all receptor subtypes except for the TP receptor, which had a lower expression in tumor tissue in 74% (32 of 43) of patients in the within group analysis as compared to increased expression in the cross-sectional samples. FP subtype receptor expression was below the detection limit in 18% of tumors (11/62), while only two patients displayed the same phenomenon in normal colon tissue. Tumor stage, grade and patient survival did not correlate to unexpressed FP subtype receptor. However, none of these patients had highly differentiated tumors and 73% of these patients (8 of 11) were males.
Figure 1. Prostanoid receptor expression in tumor tissue (n = 62) compared to normal colon tissue (n = 43). * p < 0.05 vs. normal colon tissue.
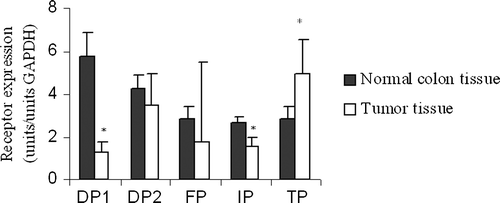
Table II. Transcript expression of PG receptors in normal colon tissue and colorectal cancers grouped according to Dukes A-D stage.
Table III. Transcript expression of prostanoid receptors in tumor tissue during progression of colorectal carcinoma.
Table IV. Transcript expression of prostanoids receptors in tumor tissue from colorectal cancers of various differentiation.
Tumor cells (HT-29 and HCA-7) did not express DP1 and IP receptor subtypes, but displayed low expression of DP2, FP and TP receptor subtypes (). Also, EP1–4 subtype receptor expression varied considerably between HT-29 and HCA-7 cell cultures ().
Table V. Estimates of prostanoid receptor expression in two established colorectal cancer cell lines with high (HCA) and low (HT-29) PGE2 production. Results are provided relative to expression levels in colon cancer tissue and as raw measures obtained by Light Cycler and Taqman determinations as indicated.
Discussion
The first reports of beneficial effects of NSAIDs in colorectal cancer patients were published several years ago Citation[7], Citation[18]. Still, the molecular basis of why and how NSAIDs inhibit tumor progression is unclear. Most reports have focused on PGE2, the major product of COX-2, and have left remaining products of COX without detailed considerations in colorectal cancer. In our earlier report we quantified PGE2 receptor expression in human colorectal tumor tissue in comparison to expression in adjacent normal colon tissue. The results revealed that high expression of EP2 receptor subtype predicted reduced disease specific survival Citation[17]. However, overall changes in expression of any other EP subtype receptor could not be related to tumor progression or tumor differentiation Citation[17]. Therefore, we have now focused on additional receptors (DP1, DP2, FP, IP, TP) for prostanoids (PGD2, TXA2, PGF2α, PGI2) produced by cyclooxygenases (COX) in order to get a more complete evaluation, since reports on the expression of prostanoid receptors in colorectal cancer are sparse or lacking in the literature.
Our results showed reduced expression in four of five prostanoid subtype receptors in Dukes A-D tumors compared to normal colon tissue, although these findings were overall most consistent for DPI and IP expression, while TP receptor expression was increased in tumor tissue (). These findings are in part a sign of imbalanced eicosanoid receptor expression in colorectal cancer tissue probably affecting tumor progression, as earlier reported for EP2–4 subtype receptors Citation[17]. However, clear cut connections or mathematical correlations to tumor stage, differentiation and progression were not observed, as found for particularly EP2 and COX-2 expression in tumor tissue predicting reduced disease specific survival Citation[17], Citation[19]. Therefore, one may anticipate more complex relationships for prostanoids in tumor carcinogenesis and progression, since altered eicosanoid homeostasis in tumor tissue is well recognized and appears a global tumor phenomenon Citation[12–14], which may decide metastatic spread Citation[16], affecting tumor angiogenesis, cell proliferation, apoptosis and immune reactions Citation[20]. However, an obvious limitation to present approach with overall tissue measurements is the risk to oversee specific alterations within or between defined cell types as tumor, endothelial- and migrating immune cells. Our recent evaluation have suggested that mathematical modeling of growth factor proteins to explain tumor progression defines different explanations accounting for prostanoids and tissue production Citation[19]. Thus, it is likely that prostanoids are important factors to define colorectal cancer progression, although it is presently not possible to present a simple and unified model.
Each prostanoid ligand and corresponding receptor has certain functions in cells and tissues; PGD2/DP1-2 are involved in the immune response, PGF2α/FP affect smooth muscle cells while PGI2/IP and TXA2/TP involve vascularity control in tissues Citation[15], Citation[21], Citation[22]; functions that are all changed in tumor tissue. The FP receptor may activate potentially oncogenic pathways such as the β-catenin transcription Citation[23] and is sometimes up-regulated in adenocarcinomas promoting neoplastic epithelial cell proliferation Citation[24]. TXA2 is involved in angiogenesis and subsequent development of tumor metastases, while PGI2 displayed anti-cancerogenic effects in a murine cell model Citation[4], Citation[25]. The DP receptor has been linked to inflammation which may represent a pre-stage to cancer, where DP1 shows anti-inflammatory effects and DP2 pro-inflammatory actions Citation[26], although PGD2 displayed anti-proliferative activity in-vitro Citation[27]. Interestingly, established cultured human colon carcinoma cell lines (HT-29, HCA-7), with low (HT-29) and high (HCA-7) intrinsic PGE2 production, expressed pro-cancerogenic receptor subtypes only as DP2, FP and TP, which are principally in agreement with reduced overall prostanoid levels in tumor tissue. Confusingly, our result on prostanoid receptor expression in HCA-7 and HT-29 cells did not entirely agree with results reported by others Citation[28], where Hawcroft et al. observed expression of DP1 in HT29 cells only without any expression of DP2 in any of five tumor cell lines including HCA-7; results apposite to ours (). This may be a question of detection limits in levels of transcripts or eventually indicates how sensitive receptor expression may be to environmental factors defined by the cell culture procedures.
In conclusion, imbalanced prostanoid receptor expression was observed in colorectal cancer without simple correlations to tumor stage, differentiation and progression. Further studies of eicosanoid receptor profiles in defined cells within colorectal tumors, obtained by fresh frozen microdissected material, are thus necessary in order to determine whether any prostanoid receptor(s) is useful as a molecular target in treatment or prevention of colorectal cancer.
Acknowledgements
Supported in parts by grants from the Swedish Cancer Society (2014), the Swedish Research Council (08712), Tore Nilson Foundation, Assar Gabrielsson Foundation (AB Volvo), Jubileumskliniken Foundation, Inga Britt & Arne Lundberg Research Foundation, Swedish and Göteborg Medical Societies and the Medical Faculty, Göteborg University, VGR 19/00, 1019/00.
References
- Cahlin C, Gelin J, Andersson M, Lonnroth C, Lundholm K. The effects of non-selective, preferential-selective and selective COX-inhibitors on the growth of experimental and human tumors in mice related to prostanoid receptors. Int J Oncol 2005; 27: 913–23
- Gelin J, Andersson C, Lundholm K. Effects of indomethacin, cytokines, and cyclosporin A on tumor growth and the subsequent development of cancer cachexia. Cancer Res 1991; 51: 880–5
- Cao Y Prescott SM Many actions of Cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol 2002;190:276–86.
- Pradono P, Tazawa R, Maemondo M, Tanaka M, Usui K, Saijo Y, et al. Gene transfer of thromboxane A(2) synthase and prostaglandin I(2) synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Res 2002; 62: 63–6
- Wang D, Dubois RN. Prostaglandins and cancer. Gut 2006; 55: 115–22
- Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: Targets for treatment and prevention of colorectal cancer?. Mol Cancer Ther 2004; 3: 1031–9
- Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: Case control results from the Melbourne Colorectal Cancer Study. Cancer Res 1988; 48: 4399–404
- Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW, Jr. Aspirin use and risk of fatal cancer. Cancer Res 1993; 53: 1322–7
- Muscat JE, Stellman SD, Wynder EL. Nonsteroidal antiinflammatory drugs and colorectal cancer. Cancer 1994; 74: 1847–54
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 2003; 348: 883–90
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev 1999; 79: 1193–226
- Yang VW, Shields JM, Hamilton SR, Spannhake EW, Hubbard WC, Hylind LM, et al. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res 1998; 58: 1750–3
- Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med 1993; 122: 518–23
- Pinto S, Gori L, Gallo O, Boccuzzi S, Paniccia R, Abbate R. Increased thromboxane A2 production at primary tumor site in metastasizing squamous cell carcinoma of the larynx. Prostaglandins Leukot Essent Fatty Acids 1993; 49: 527–30
- Ushikubi F, Sugimoto Y, Ichikawa A, Narumiya S. Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn J Pharmacol 2000; 83: 279–85
- Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res 2006; 66: 9794–7
- Gustafsson A, Hansson E, Kressner U, Nordgren S, Andersson M Wang W, et al. EP1-4 subtype, COX and PPARgamma receptor expression in colorectal cancer in prediction of disease-specific mortality. Int J Cancer 2007;121:232–40.
- Thun MJ, Namboodiri MM, Heath CW, Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991; 325: 1593–6
- Cahlin C, Lönnroth C, Arvidsson A, Nordgren S, Lundholm K. Growth associated proteins in tumor cells and stroma related to disease progression of colorectal cancer accounting for tumor tissue PGE2 content. Brit J Surg (submitted 2007).
- Lönnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H. Lundholm K Preoperative treatment with NSAID increased tumor tissue infiltration of activated immune cells in colorectal cancer. Submitted 2007.
- Hirai H, Tanaka K, Takano S, Ichimasa M, Nakamura M, Nagata K. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol 2002; 168: 981–5
- Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel AB, et al. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: Consequence on the polarization of naive Th cells. J Immunol 2003; 170: 4943–52
- Fujino H, Regan JW. Prostanoid receptors and phosphatidylinositol 3-kinase: A pathway to cancer?. Trends Pharmacol Sci 2003; 24: 335–40
- Sales KJ, Milne SA, Williams AR, Anderson RA, Jabbour HN. Expression, localization, and signaling of prostaglandin F2 alpha receptor in human endometrial adenocarcinoma: regulation of proliferation by activation of the epidermal growth factor receptor and mitogen-activated protein kinase signaling pathways. J Clin Endocrinol Metab 2004; 89: 986–93
- Nie D, Lamberti M, Zacharek A, Li L, Szekeres K, Tang K, et al. Thromboxane A(2) regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem Biophys Res Commun 2000; 267: 245–51
- Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, Nagase H, Ishii A, Matsushima K, et al. Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2. Clin Exp Allergy 2004; 34: 1283–90
- Yoshida T, Ohki S, Kanazawa M, Mizunuma H, Kikuchi Y, Satoh H, et al. Inhibitory effects of prostaglandin D2 against the proliferation of human colon cancer cell lines and hepatic metastasis from colorectal cancer. Surg Today 1998; 28: 740–5
- Hawcroft G, Gardner SH, Hull MA. Expression of prostaglandin D2 receptors DP1 and DP2 by human colorectal cancer cells. Cancer Lett 2004; 210: 81–4
