To the Editor
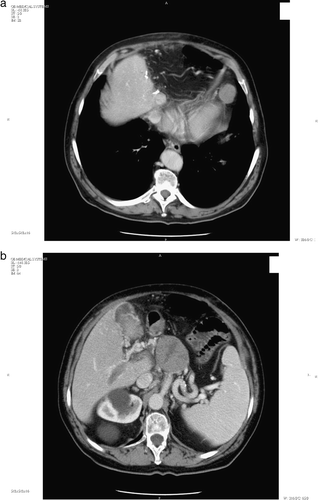
In April 2003, a 70-year-old man with chronic hepatitis C came to our attention because of sudden Alpha-feto-protein (AFP) increase (more than 20.000 ng/ml). A CT scan showed a portal thrombosis, multiple hepatic lesions, hilar node, and pericardial fat-tissue node. (a,b). In 2001 he underwent a Trans-Arterial-Chemo-Embolization (TACE) and in December 2002 surgical resection of the II and III liver segments, with a pathology report of a 7.5 cm G3 HCC with portal neoplastic thrombosis.
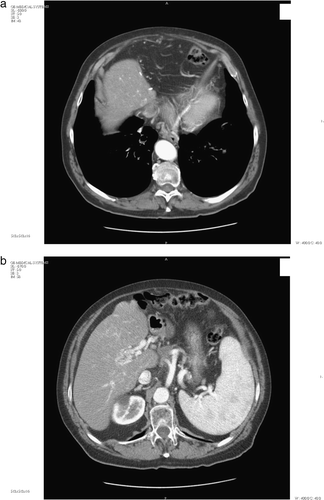
Systemic treatment with UFT, five capsules a day (one 100-mg capsule = Tegafur 100 mg + Uracil 224 mg) over 4 weeks every 5 was started. An AFP reduction and tumor shrinkage concurrently occurred. The most frequent toxicities were G3 transaminitis, G1 hyperbilirubinemia, G1 thrombocytopenia, and G2 leuconeutropenia that led to a dose reduction of UFT to 3 capsules a day four weeks on one week off. A normal value of AFP was observed in February 2004. In May 2005, the CT scan showed the best response characterized by disappearance of the pericardial lymph node and around 90% reduction of the celiac node (a,b). In December 2005 UFT was withdrawn due to radiological and biochemical progression of disease. Then the patient received Thalidomide 200 mg/day, within a trial, until December 2006, and cyclophosphamide and methotrexate as a “metronomic” schedule until now. He is currently alive and asymptomatic, with a slight biochemical and radiological progression of disease.
Discussion
Survival rate of patients with advanced HCC is poor, with 20% at 1 year and less than 5% at 5 years Citation[1]. Radical surgery is the only potentially curative treatment. However, only a very small percentage of patients can benefit from it. The vast majority of patients are unsuitable for surgery and receive a palliative treatment.
A lot of chemotherapeutic agents have been used in HCC patients, but none of them has clearly proved effective in terms of survival. Moreover, even when effective on the tumor chemotherapy can be detrimental for the patient due to its toxicity. Finally, in many cases chemotherapy cannot be proposed due to severity of underlying comorbidity.
UFT is a combination of tegafur (a 5-FU-prodrug) and uracil (a competitive inhibitor of 5-FU catabolism, at the level of dihydropyrimidine dehydrogenase or DPD) in a molar ratio of 1:4. Its activity is usually related to the combination with leucovorin. However, we decided not to add leucovorin to reduce the risk of toxicity Citation[2]. Our patient carried on UFT over a total of 30 months, without severe toxicity and never limited in his daily life activity.
Our case report shows that it is possible to successfully treat patients with advanced HCC, with no impact on quality of life and long-lasting control of disease growth, using low dose of an oral chemotherapeutic agent. The low dose and avoidance of leucovorin make UFT more tolerable and manageable and less expensive, without reducing its activity. This experience prompted us to study the effectiveness of metronomic oral chemotherapy in HCC cirrothic patients.
Acknowledgements
Special thanks to Nordiana Baruzzi for her kind cooperation in revising the English in this manuscript.
References
- Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 2005; 20: 1781–7
- Hoff PM, Pazdur R. UFT plus Leucovorin: A new oral treatment for colorectal cancer. Oncologist 1998; 3: 155–64