Abstract
Background. Most studies regarding esophageal cancer are based on a selection of patients, influencing the prognosis as well as other variables measured. Sweden may be unique in that it has registries that cover the whole population, permitting population based studies regarding diseases such as esophageal cancer. This also makes it possible to study the true nature of a population of patients and to describe changes in that population over time. Method. Retrospective analysis of the files of all 1284 patients diagnosed with esophageal cancer in Stockholm County 1978–1995. The study period was divided into three six-year intervals (periods I, II and III). Results. A total of 201 patients were diagnosed at autopsy. They were only analyzed regarding histopathological and demographic parameters. A statistically significant increased survival for the whole group of patients was found, but this improvement in survival was not found among resected patients. No survival benefit was noted for patients operated on at large centers compared to patients operated on at surgical clinics with few yearly resections performed. The well-known increase in the incidence of adenocarcinoma in the esophagus among men was documented. A tendency (non-significant) of an increase in the incidence of adenocarcinoma among women was also noted. Conclusions. Survival seems to have increased among esophageal cancer patients, but this survival benefit is not dependent on improved surgery. The number of yearly operations in a clinic did not correlate to long-term survival in this study.
Esophageal cancer has a poor prognosis Citation[1–3] and is associated with severe symptoms that quickly deprive the patients of various aspects of their quality of life. Most studies assessing the outcome of various treatments for esophageal cancer are based on a selection of patients. This influences the prognosis as well as other variables measured. Sweden may be unique in that since 1686, it has a population registry that covers the whole population. This complete coverage even applies to the general Cause of Death Registry and the Swedish Cancer Registry. These registries, as well as a relatively low migration tendency in the population, make Sweden ideal for population-based studies regarding the incidence, treatment and outcome of malignant diseases such as esophageal cancer. Sweden represents a low incidence area regarding esophageal cancer, the annual incidence being approximately 3.9/100 000 (Swedish Cancer Registry), including both main histological types. This study describes the true incidence and treatment of the disease in the population of the County of Stockholm (1.7 million inhabitants) from 1978 to 1995 with a follow-up of patients through December 2000.
In older series in the West 90% of esophageal cancer was of the squamous cell type. The world-wide incidence of squamous cell carcinoma has remained constant Citation[4], but there has been a shift towards the other major type – adenocarcinoma – during the last decades. In the United States adenocarcinoma of the esophagus shows the fastest increasing incidence of any solid cancer in white men Citation[5], with about 50% of newly diagnosed esophageal cancers now being adenocarcinoma. The same tendency has been noted in Western Europe Citation[6], Citation[7]. This is also true for Sweden, where even a rise in the incidence of squamous cell carcinoma has been registered, however less pronounced Citation[8].
The aim of this study was to investigate the nature of the population of esophageal cancer patients in Stockholm county during 1978–1995, describing changes in incidence, demographic parameters, survival, treatment policies and results.
Patients and methods
Stockholm county consists of 25 different communes with a total population of 1.7 million. Partly because of a continuing urbanization process in Sweden since the end of the 19th century, the population has increased from 1 519 000 to 1 726 000 during the inclusion period.
All patients found in the esophageal cancer file of the database of the central Cancer Registry regarding Stockholm county during the period 1978 to 1995 were evaluated. Using the Central Registry of Inpatient Care in Stockholm county, all patients’ hospital admissions including admissions to geriatric clinics were noted. Files were evaluated after permission from the central head of the Stockholm County Archives and the respective chiefs of staff of the various clinics where patients had been treated. If the patients were not alive, the cause of death was found either in the files or in the central Cancer Registry. Only patients with a histopathological diagnosis of cancer in the esophagus were included into the study.
Definitions
Esophageal cancer
The diagnosis of esophageal cancer is easy in most cases, but the discrimination between a distal esophageal adenocarcinoma and a cardiac tumour is still problematic and a common cause of debate Citation[9]. In this retrospective study, a detailed histopathological classification including the evaluation of metaplasia, was not possible. In our study adenocarcinoma is classified as esophageal cancer if the bulk of tumour was found above the gastro-esophageal junction. All squamous cell carcinomas in the esophagus, with the bulk of tumour below the cricopharyngeal muscle, were also classified as esophageal cancers.
Cause of death
In the Cancer Registry up to two causes of death were registered according to ICD7 or ICD9 during the inclusion period. If the esophageal cancer was registered as one of these two causes of death, the malignancy was regarded as causing the mortality.
Analysis and statistical considerations
Patients where the diagnosis was established at autopsy were excluded from analysis of clinical parameters, but they were included in the analysis regarding demographic and histopathological data.
In an effort to evaluate changes over time, three different six-year time periods, 1978–1983 (period I), 1984–1989 (period II) and finally 1990–1995 (period III), were analyzed. Survival time was calculated from the day of diagnosis with the Kaplan Meier method. For comparisons between different survival curves, the log-rank test was used. Trends over time were analyzed with logistic regression analysis. Other comparisons between groups were made using the χ2 test. If more than 10% of the data was missing for a particular parameter, no further calculations were made regarding the data.
Ethical considerations
The study was approved by the ethical committee of the Karolinska Institute according to the World Medical Association's Declaration of Helsinki, 1964, and the Amendment of Tokyo in 1975. All living patients gave informed consent prior to participation in the study.
Results
Registration accuracy
Initially, 1366 patients registered as esophageal cancers were found in the Cancer Registry. After a close evaluation of the files, the diagnosis proved to be incorrect in 82 individuals (6%) and they were excluded from further analysis. Records of patients registered with cardiac cancers in the Cancer Registry were evaluated to identify esophageal cancers that were wrongly registered as cardiac tumours.
Patients
Sex
In Sweden the male to female ratio for esophageal cancer is approximately 3:1 (Swedish Cancer Registry). Out of the 1284 patients in this study, 359 (28%) were women, resulting in a male to female ratio of 2.6:1. The corresponding figures for the two major histologic types show a male to female ratio of 2.5:1 in squamous cell carcinoma (n = 988) and 3.5:1 in adenocarcinoma (n = 193).
Age
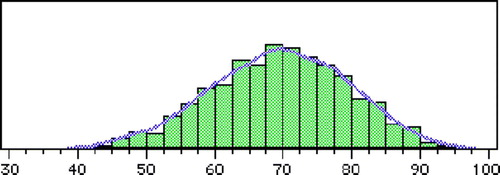
The average age at the time of diagnosis was 69.3 years (range 30–99) (). Age did not change over time. A difference between the sexes was found with male patients diagnosed at an average age of 68.1 compared to women at 72.5 (p < 0.001). This difference between the sexes was consistent, regardless of histological type. Squamous cell carcinomas were diagnosed at an earlier age than adenocarcinomas (68.8 vs. 70.0 years, not significant (n.s.)).
Figure 2. Age-specific incidence (cases/100 000) of esophageal cancer in Stockholm County 1978–1995. (n = 1284)
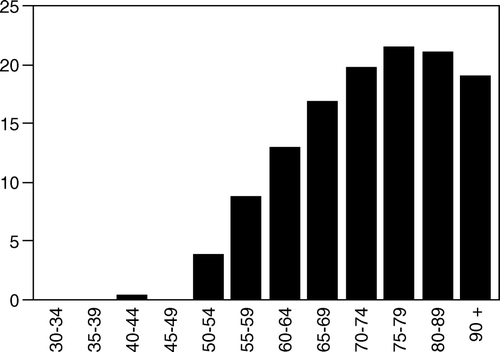
Figure 3. Age-specific incidence (case/100 000) of esophageal cancer in Stockholm County 1978–1995 for three age groups.
The average age of individuals where the diagnosis were made at autopsy, was 6.4 years more than in living patients (74.9 vs. 68.5, p < 0.001).
Autopsy
Of the initial 1284 patients, 201 (16%) were diagnosed at autopsy. This figure included patients with an in vivo suspected, but not histopathologically verified, esophageal cancer as well as patients with an incidental tumour found at autopsy.
The patients diagnosed at autopsy were only analyzed regarding demographic and histopathological parameters. Hence there were 1083 patients left for the clinical evaluations.
Dysphagia and weight loss
The median duration of dysphagia prior to time of diagnosis was 3 months (range 0–48) for the whole period. No differences in time intervals for the three different time periods were found. A total of 485 patients (45%) had experienced symptoms of dysphagia more than 4 months prior to diagnosis. The dysphagia was classified as shown in . Notably 141 patients (13%) did not experience any or only minor dysphagia pre-diagnosis. Dysphagia and associated symptoms resulted in an average weight loss of 8.3 kg (range 0–38) during the 6 months preceding diagnosis. Notably 154 patients (14%) did not lose any weight.
Table I. Dysphagia among esophageal cancer patients in Stockholm County 1978–1995.
Tumours
Histological type
In this series 77% of tumours were squamous cell carcinomas while 15% of the patients had adenocarcinomas. Seven percent of tumours were undifferentiated. Three leiomyosarcomas, one undifferentiated sarcoma and one lymphoma were found. One case of adenosquamous cancer, with obvious features from both major histological types, was identified. ().
Table II. Histological subgroups among esophageal cancer patients in Stockholm County 1978–1995. (n = 1284)
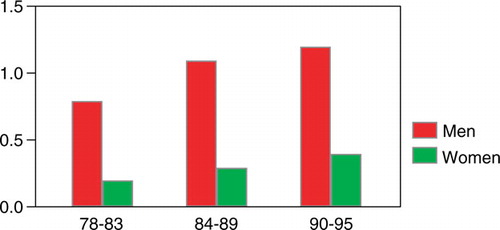
During the study period the total incidence of esophageal cancer remained constant, but there was a tendency for a decreasing incidence among men attributable to a lower incidence of squamous cell carcinoma (4.80–3.94/100 000) in the male population (n.s.)). The opposite was noted for women, where an increase in incidence was found for both major histological types (n.s.) ().
Figure 4. The incidence of esophageal cancer/100 000 in Stockholm County 1978–1995. The incidences of the major histological types are indicated.
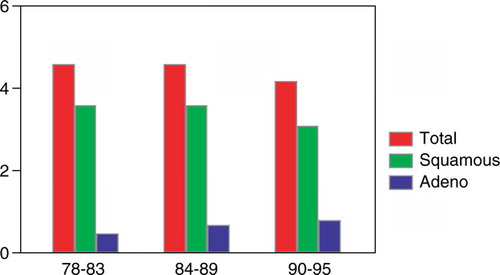
Figure 5. The incidence of squamous cell carcinoma of the esophagus/100 000 among men and women in Stockholm County 1978–1995.
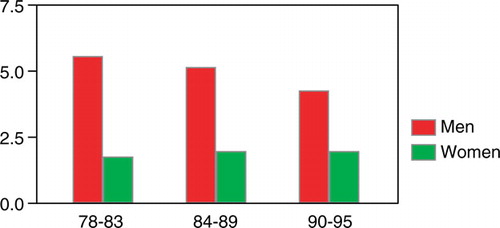
Figure 6. The incidence of adenocarcinoma of the esophagus/100 000 among men and women in Stockholm County 1978–1995.
The relative incidence of adenocarcinoma increased from 11.2% during time period I through 15.1% in time period II to 18.7% during the last 6 years. This increase was statistically significant (p < 0.01) and it was mainly found among men (p < 0.01). An increase was also found among women. This increase was linear, but did not reach statistical significance ().
Differentiation
Only 6% of the tumours were classified as highly differentiated, while 44% and 43% were classified as moderately and poorly differentiated respectively. Seven percent were undifferentiated cancers.
Location
Twenty percent of tumours were located in the upper third of the esophagus (≤24 cm from the incisors), 50% in the middle (>24– ≤ 32 cm from the incisors) and 30% in the lower third. Most adenocarcinomas (51%) were found in the lower esophagus and only 3% were located in the upper third.
Twenty-six patients (2.4%) had synchronous tumours. Twenty-one patients with squamous cell carcinoma had two or more squamous cell carcinomas of which 11 were located in the esophagus and ten in the oral cavity or pharynx. Four patients with epidermoid cell carcinoma had five synchronous adenocarcinomas (3 cardiac and 2 gastric carcinomas). In one patient with adenocarcinoma two separate tumours were found.
Tumour stage
During the major part of the time period studied, no reliable preoperative staging method was generally available. Notably, however, with the investigations available, in 99 patients (9%) distant metastases were found at the initial evaluation and 156 patients (14%) had local overgrowth of tumour on other organs. In the first six-year period studied, 47 patients (14%) were found to have advanced disease corresponding to T4 and/or M1 according to the TNM classification. Seventy (70) such patients (19%) were found during period II and 76 (20%) during period III.
Diagnosis
Where?
Most patients that were alive were diagnosed at ear, nose and throat (ENT) clinics (48%), since patients with dysphagia traditionally had been referred to these clinics. Thirty-seven percent were diagnosed at surgical clinics while private endoscopists diagnosed 9%.
During period I of the study 64% of the living patients were diagnosed at ENT centers compared to 26% at surgical clinics. The corresponding figures for period III were 33% and 51% respectively. This illustrates a shift in the flow of patients from ENT departments to surgical clinics (p < 0.001).
Of the 201 patients diagnosed at autopsy, 146 (73%) were hospitalized in geriatric clinics prior to death.
Treatment
Surgery with a curative intent
A total of 331 patients were explored with curative intention, equaling an operation rate of 31%. No difference in operation rate was found between hospitals. However, 67 patients (20%) were found to be unresectable at operation. The total resection rate was 24%. The number of operations increased from 29% of the esophageal cancer population in period I to 37% in period III (p < 0.05). The resection rate of the operated patients also increased during the time periods from 77% during periods I and II to 83% in time period III (n.s.). Thirty day mortality for the resected patients was 8%. This figure dropped from 12% during period I through 10% in period II and finally to 6% during period III (n.s.).
In 91% of resected patients an Ivor-Lewisoperation was performed using the stomach as esophageal substitute in 97% of cases. In eight patients colon interposition was used. Eighteen resections were performed transhiatally as described by Orringer Citation[10]. In three patients with high tumours laryngectomy was performed.
During the whole study period 11 different surgical clinics performed esophageal resections. The number of operating clinics per year varied from three to eight. Consequently, the number of clinics operating more than five patients per year was zero during 7 of the first 12 years of the study. A concentration process of the surgical management of esophageal cancer patients took place during 1978–1995 resulting in three operating surgical clinics at the end of the study period.
Oncology with curative intent
Twenty-three percent (245) of the patients were initially planned to receive preoperative oncologic therapy (radiotherapy 11%, radiochemotherapy 11%, chemotherapy 1%) mainly due to advanced disease. Only 57% of these planned treatments were actually completed.
In this study chemoradiation was administered according to two different protocols. Before 1990 a neoadjuvant bleomycin-based regimen with 30–35 Gy of radiation was used in 60 patients. In the 1990's a cisplatinum-based neoadjuvant protocol with 40 Gy of radiation was used in 56 patients. This was the first neoadjuvant protocol routinely used also for adenocarcinoma patients. The median survival following bleomycin-based treatment was 7.8 months compared to 13.3 months in the cisplatin group. Only 57% of patients in the bleomycin group received the full treatment, compared to 77% of patients treated with cisplatinum.
Nine percent of patients were initially planned for curative full dose radiotherapy. It was administered to 80% of patients planned for the treatment, probably illustrating a higher compliance with a less demanding therapy, compared with neoadjuvant oncology regimes.
Six percent of the patients were planned for full dose radiochemotherapy. The therapy was not completed in 42% of these patients due to treatment-related morbidity (17%) and progression of disease (25%).
Palliative oncology
Palliative oncology was planned as the primary treatment in 27% of patients of which 75% actually received treatment. Thirty-nine percent of patients received palliative radiation therapy (30–46 Gy), 26% palliative chemotherapy and in 10% radiotherapy and chemotherapy was combined. However, only 63% of patients could fulfill palliative chemoradiation compared to 80% for the less demanding palliative radiation. The palliative chemotherapy consisted, as the curative intended chemotherapy, mainly of bleomycin based treatments during time periods I and II, while cisplatin-based regimens were used during time period III. In 1995 Gemzar was introduced as a palliative chemotherapy treatment of esophageal cancer.
Palliative surgery and endoscopy
Almost all (98%) of the 331 patients planned for surgical and/or endoscopic palliative therapy received the planned treatment. Surgical palliation consisted of operative gastrostomy (n = 92). No palliative resections were planned preoperatively and no bypass procedures were performed. With the advent of routine endoscopy, a number of palliative techniques were introduced. Seven patients were treated with repeated dilatations as the only therapeutic method. This treatment was soon abandoned as a palliative treatment because of the short duration of effect. Initial endoscopic ablation techniques included alcohol injections (n = 1) and electric cautery treatments (n = 1). These treatments were replaced by YAG-laser ablation in1992 (n = 29). Laser treatment often required repeated sessions (average amount of 2.8 sessions, range 1–10) at short time intervals. The dominating endoscopic palliative treatment was stent placement (n = 127). During the first two time periods Celestine or Atkinson tubes were used. These rigid rubber or silicone tubes required general anesthesia and occasionally laparotomy. The procedures, often performed on patients in poor conditions, were associated with a considerable morbidity. During the last time period, rigid stents were replaced by expandable metal stents that could be placed endoscopically-guided in lightly sedated patients. The difference in morbidity becomes evident in the post-treatment one-week mortality. Eight of the 78 patients (10%) that were treated with rigid tubes died within seven days, compared to one of 49 patients (2%) that received metal stents (n.s).
Survival
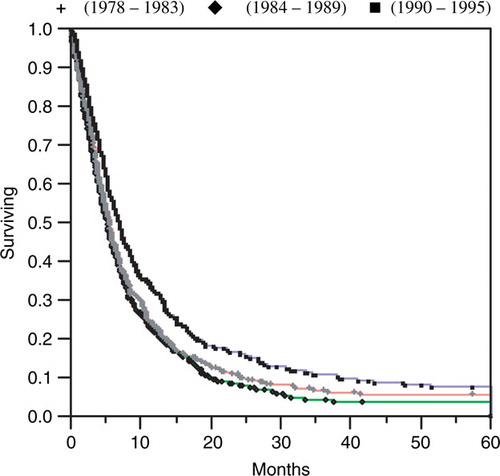
The median disease-specific survival for all non-autopsy patients was 5.8 months (). This survival increased from 5.7 months during time period I to 6.9 months during time period III. The total disease-specific five-year survival was 6%. This figure increased during the study from 5.8% during time period I to 8.2% during time period III, where the worst survival figures were found in the second time period with a median disease-specific survival of 5.4 months and a five-year survival of only 3.8%. All these differences in survival were statistically significant (p < 0.05) ().
Figure 7. Disease-specific survival among esophageal cancer patients in Stockholm County 1978–1995 according to the Kaplan Meier method.
The total disease-specific median survival of women was 7.2 months compared to 5.4 months for men (p < 0.01).
The older half of patients had a median survival of 5.3 months compared to 6.6 months for the younger patients (p < 0.01).
Adenocarcinomas had a better prognosis, with a median survival of 7.1 months compared to 5.7 months for the squamous cell group (p < 0.05).
Differentiation grade had no statistically significant impact on survival.
Tumours in the upper and middle thirds of the esophagus both had a median survival of 5.6 months compared to 6.8 months for lower esophageal tumours (p < 0.05). This difference in median survival is maintained when only squamous cell carcinomas are considered (5.4 and 5.6 months vs. 6.7 months respectively (p < 0.05)).
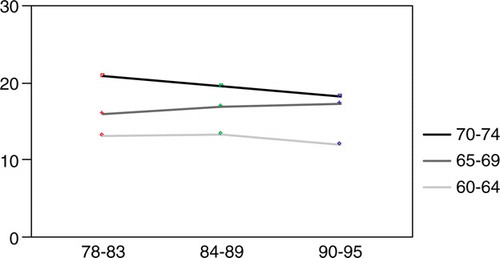
The median disease-specific postoperative survival for resected patients was 12.2 months. This figure increased from 9.8 months during period I through 10.9 months in period II to 13.4 months during the last 6 years of the study (). However, there were no statistically significant differences in postoperative survival between the three groups according to Kaplan-Meier analysis. There was a tendency (n.s.) of an early survival benefit during the first postoperative year for the patients in the last time period, but this tendency disappeared with a longer follow-up. Twenty-one percent of the resected patients had a survival exceeding 5 years.
Figure 8. Disease-specific survival among resected esophageal cancer patients (n = 264) in Stockholm County 1978–1995 according to the Kaplan Meier method.
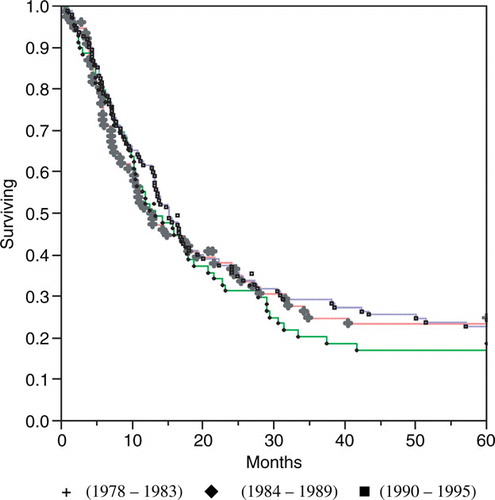
No survival differences were found between the surgical clinic with the largest number of resections (n = 100), the four largest clinics (ntot=212) and the seven clinics with the smallest number of resections (ntot =52) as seen in median postoperative survival times of 11.0, 12.1 and 12.3 months respectively, or postoperative mortality (8.0, 8.5 and 8.0% respectively).
The median survival for non-operated patients treated with full dose radiotherapy was 10.6 months. This figure was the same in the first and last time periods (11.4, n = 32 and 17 respectively), but lower during time period II (8.4, n = 29).
Patients treated with full dose radiochemotherapy (n = 35) had a median survival of 10.9 months.
Neoadjuvant radiochemotherapy treatment (n = 75) resulted in a median survival of 18.1 months with a five year survival of 27.3%. The low numbers in periods I and II (n = 17 and 6) do not permit a calculation of changes in survival over time following these treatments.
Symptomatic effects of palliation
Less than half (44%) of the patients treated with palliative radiation therapy actually experienced symptom relief. If palliative radiation was combined with chemotherapy (n = 27) 59% of the patients experienced symptom relief, including two cases of clinical complete responses. With only chemotherapy as palliative treatment, 25% of patients experienced symptom relief.
Seventy-two percent of the stented patients experienced improved swallowing following stent application. There was no difference regarding symptom relief between patients treated with rigid tubes compared to newer metal stents. Twelve percent of patients with metal stents experienced chest pain, which was often difficult to treat. Pain or lack of pain following rigid tubes was not documented well enough to permit any conclusions. As mentioned above, a higher postoperative mortality within the first week was noted for the rigid tube group, indicating a higher complication rate (n.s.). One patient, with an uncovered nitinol stent, died of mediastinitis eleven months after stent placement. This was due to a stent fracture causing a perforation of the esophagus.
Twenty-nine patients were treated with laser. Of these, 75% experienced a reduction of dysphagia, but two perforations were registered. Both were successfully managed conservatively.
Causes of death
In total, 94% of patients died with esophageal cancer. It is of interest that 15 patients died of esophageal cancer more than 5 years after diagnosis. These patients may have had new primary tumours, but well documented metastases as the only manifestation of tumour were registered.
Thirty-three patients died without any manifestations of esophageal cancer. Half of these deaths (n = 15) were caused by atherosclerosis-related diseases (thromboembolism in 5, ischemic heart disease in 9 and aortic aneurysm in 1). Seven patients died of other malignancies (ENT related cancers in 4, lung in 2 and pancreas in 1). Many patients had a history of ethanol abuse. This resulted in two deaths caused by ethanol intoxication, two cases of liver failure and two fatal accidents and one fatal case of pulmonary tuberculosis. Respiratory failure as a complication of smoking-related disease was found in XX patients. In two patients pneumonia and in one patient senility was regarded as the cause of death.
A number of contributing causes of death were registered among the patients who died with esophageal cancer. The most common was pneumonia, probably related to aspiration and/or tracheoesophageal fistulae. Three percent of these patients died with other synchronous malignancies. Sixty-three percent of these tumours were found in the ENT region in squamous cell epithelium.
Forty-six patients (5%) had contributing causes of death directly related to alcoholism. These include intoxication and trauma as well as liver failure and bleeding esophageal varicose veins.
Discussion
Esophageal cancer is associated with a dismal prognosis and severe symptoms. The majority of patients in the West are diagnosed at a late stage, when locoregional therapies such as surgery and radiation cannot offer cure. Furthermore, the majority of patients considered for curative therapy eventually die of esophageal cancer.
Even in this study of the esophageal cancer population in Stockholm a disappointing 6% of patients were living 5 years after diagnosis. However, an improvement in five-year survival was found during the study period, from 5.8% in 1978–1983 compared to 8.2% in 1990–1995 (p < 0.01). The mortality was not corrected for changes in mortality figures in the general population. Therefore, a general improvement in health may have influenced survival figures. However, the survival figures of the study are disease-specific and we do believe that the overwhelming impact of esophageal cancer dominates the survival figures in a way that external factors not related to the disease or to the treatment of the disease, are of less importance.
Different strategies have been tried to overcome the poor treatment results. More radical surgery Citation[11] with extensive lymph node dissection is now standard treatment in some surgical units. These operations increase postoperative morbidity, but no randomized trials have, to our knowledge, proved the benefit of these surgical techniques.
This study illustrates the fact that surgery has been considered the standard curative treatment of esophageal cancer. At the end of the study the majority of patients were initially evaluated in surgical clinics. One feature that may have influenced the improved survival figures was decreasing postoperative 30-day mortality during the study period, from 12% during period I to 6% in period III. This decrease is however not statistically significant.
The relative numbers of operations, as well as resection rates, increased significantly during the study period. This may illustrate improved staging technology, where more operations, but fewer explorations on more advanced cases, were performed. During the first time period general use of endoscopy was introduced. During time period II computed tomography (CT) became available, as did endoscopic ultrasound during time period III. A wider use of neoadjuvant chemoradiation with a possible downstaging of tumours may also have had an impact on the increased number of operations and resections during time period III. The probability that more advanced tumours were operated on during the last time period may influence postoperative survival.
The findings in this study that the number of resections performed in a clinic have no impact on survival or postoperative mortality is surprising. It is also contradictory to findings in other studies regarding esophageal cancer, where it has been shown that a well-trained surgeon gets better results Citation[12], Citation[13]. It may be speculated that in large centers many different surgeons perform the same operation compared to only one surgeon in smaller units. If that is true, the number of operations performed at a clinic does not necessarily indicate the operating surgeon's clinical experience. If this is true, the number of operations performed in a clinic does not necessarily indicate the operating surgeon's clinical experience. This hypothesis is supported by recent findings from the SECC-registry in Sweden, where the number of resections performed in a center did not influence survival Citation[14]. A high resection volume for the individual surgeon did however improve the results.
Postoperative 30-day mortality for the resected patients was 8%. This figure dropped from 12% during period I through 10% in period II and finally to 6% during period III (n.s.). This non-significant difference is, probably because of small numbers.
Radiotherapy, as surgery, is also a locoregional therapy. It is of interest that long-time survival following radiotherapy alone in large historical series seems to be about the same as survival following surgery alone Citation[1], Citation[2]. This, in spite of the possibility that these historical oncologically treated patients represent a negative selection of patients compared to surgical cases during the same time period. The reason for this assumption is that surgery has always been the standard curative treatment option. Hence oncology has been regarded as the second line of treatment reserved for patients unfit for surgery.
Radiotherapy has also been used as an adjunct to surgery, either pre- or postoperatively. In the Second Scandinavian Trial in Esophageal Cancer, Nygaard et al. found a survival benefit of preoperative radiotherapy, after pooling of patients Citation[15]. Other authors have not confirmed these results Citation[16], Citation[17]. Some of the patients in our study took part in the Second Scandinavian trial.
Preoperative radiotherapy, either alone or combined with chemotherapy, was only completed in 57% of patients where it was planned. The main reason for this was progress of disease during therapy. This illustrates a poor effect of radiation therapy that may have resulted in treatment delay, as radiation is often administered over several weeks. This may have influenced final results. It is also of interest that only 44% of patients receiving palliative radiotherapy experienced an improvement of dysphagia.
Most patients suffer from a disease that is no longer locoregional at the time of diagnosis. Locoregional treatments such as surgery and/or radiotherapy cannot offer cure to these patients.
Chemotherapy is systemic, in theory reaching all cells in the body. Different chemotherapy protocols have been tried in esophageal cancer, either as single therapy Citation[18], but more often as part of a multimodal treatment regime. The results regarding cure have been poor until the last decade when cisplatinum based chemoradiation, either in full dose regimens or in neoadjuvant protocols was introduced Citation[19–21]. These treatments resulted in complete response figures exceeding 40% in some series. An improved survival for complete responders was recorded in several trials. The general impact on treatment strategies is however not yet established if these neoadjuvant protocols are compared to the standard treatment, i.e. surgery. Randomized prospective studies differ regarding conclusions of survival for patients receiving preoperative chemoradiation.
In this study two different chemoradiation protocols were used. The early bleomycin-based protocol resulted in a poorer compliance and a shorter median survival than the later cisplatinum-based regime.
In the 1990's a cisplatinum-based neoadjuvant protocol with 40 Gy of radiation was started. Fifty-six patients received this treatment in our study. This was the first neoadjuvant protocol regularly used also for adenocarcinoma patients. Until then, adenocarcinomas were considered to be relatively resistant to chemotherapy, but this opinion was subsequently challenged.
Neoadjuvant radiochemotherapy treatment (n = 75) resulted in a median survival of 18 months compared to 12 and 11 months for surgery and full dose radiation respectively. It must be taken into account that the demanding preoperative chemoradiation treatment therapy may represent a selection of more healthy patients, compared to the other two treatment modalities, since the treatments were not compared in a prospective randomized setting.
We do not believe that further development of surgery will substantially increase survival figures, since the disease at the time of diagnosis is often disseminated and beyond cure with locoregional treatment modalities. The future probably lies in the further development of systemic treatments, including chemotherapy and immunotherapy such as antineoangiogenetic agents. It must however be remembered that surgery offers cure in a high percentage of cases in the early stages of esophageal cancer. In more advanced local disease, resection is often in reality palliative, but does avoid disabling dysphagia in a large percentage of patients.
Earlier detection of tumours will doubtlessly improve treatment results. In this study the symptomatic time period before the diagnosis was established, did not decrease during the study period. This indicates that awareness in the population regarding alarm symptoms such as dysphagia does not seem to have increased in spite of increased public information aimed at early detection. Forty-five percent of patients had experienced dysphagia for at least four months prior to diagnosis. This may represent a clinically important delay. It was not possible in this study to differentiate between doctor's and patient's delay regarding diagnosis. It must however be remembered that dysphagia is a late symptom in esophageal cancer.
Screening programs based on the identification of risk factors in epidemiological studies are aimed at identifying tumours at an early stage. Gastroesophageal reflux symptoms have been identified as a risk factor for adenocarcinoma of the esophagus Citation[22]. Since gastroesophageal reflux symptoms are common, surveillance of these patients seems unrealistic. The possible endstage of gastroesophageal reflux disease (GERD), Barrett's esophagus, is an established risk factor for adenocarcinoma and surveillance programs are general practice. The benefits of these programs are under discussion. One reason for this is that only one of twenty cases of Barrett's esophagus in the population are discovered and subjected to surveillance Citation[23]. In Western countries tobacco and alcohol abuse are established risk factors for squamous cell carcinoma of the esophagus. The disease is, however, still rare in alcoholics and surveillance programs are not feasible.
Most patients, including cases treated with curative intent, will eventually die of esophageal cancer. This means that the dominant treatment goal will be good palliation. The leading symptoms in esophageal cancer are related to dysphagia. The major aim of palliation is therefore relief of dysphagia. In the first time period of this study palliation could be achieved through radiotherapy or through the application of rigid tubes. Palliative radiotherapy was effective in less than half of the cases and it required several weeks before an evaluation of the effect was possible. The tubes had an instant effect but they needed general anesthesia and, in the beginning, a laparotomy for placement. The complication rate was high and often the final, and only, treatment was an operative gastrostomy.
Six percent of the initially investigated patients in the cancer registry were wrongly identified as esophageal cancers. This number is high enough to have a theoretical impact on conclusions drawn from information available in the registry. The bulk of these patients had gastric or cardiac cancers, but a number of pulmonary neoplasms and even patients with esophagitis were wrongly included. In most of these cases the registration was made on initial information, where later evaluations changed the diagnosis. However, the initial registration remained unchanged in the registry.
A total of 201 patients were diagnosed at autopsy. The older age of this group of patients indicated patients with poorer general health, where diagnostic efforts may have been limited. This may also be illustrated by the number of autopsies performed in patients that died in geriatric clinics (n = 146).
In the first six-year period studied, 47 patients (14%) were found to have advanced disease, corresponding to T4 and/or M1 in the TNM classification. Seventy such patients (19%) were found during period II and 76 (20%) during period III. In time period I significantly fewer patients with advanced disease were found (p < 0.05) compared to the following periods. These three time periods correlate to the introduction of three new diagnostic tools in wider clinical practice, i.e. endoscopy during time period I, CT in period II and finally endoscopic ultrasound during period III. Better diagnostic procedures may have increased the relative number of advanced tumour stages identified correctly preoperatively.
Adenocarcinomas had a better prognosis, with a median survival of 7.1 months compared to 5.7 months for the squamous cell group (p < 0.05). It must however be noted that almost half (44.3%) of the adenocarcinomas were diagnosed in time period III when survival had generally improved for the whole group. If only time period III was considered there was no significant difference in survival between the two major histological types.
Hansson et al. found an increase in the incidence of both major histological types of esophageal cancer in Sweden Citation[8]. This increase was only found among men. In our study a constant total incidence of esophageal cancer was found. An increase in the incidence of adenocarcinoma among men was noted, but also, interestingly, non-significant increase in the incidence of adenocarcinoma among women. This tendency among female patients is found also in squamous cell carcinoma and continues through the investigated three time periods. This finding demands further epidemiological studies.
References
- Earlam R, Cunha-Melo JR. Esophageal squamous cell carcinoma I: A critical review of surgery. Br J Surg 1980; 67: 381–90
- Earlam R, Cunha-Melo JR. Esophageal squamous cell carcinoma: II. A critical review of radiotherapy. Br J Surg 1980; 67: 451–61
- Müller JM, Erasmi H, Steltzner M, Zieren U, Pichlmaier H. Surgical therapy of esophageal carcinoma. Br J Surg 1990; 77: 845–57
- Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer 1995; 75: 154–70
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991; 265: 1287–9
- Hansen S, Wiig JN, Giercksky KE, Tretli S. Esophageal and gastric carcinoma in Norway 1958–1992: Incidence time trend variability according to morphological subtypes and organ subsites. Int J Cancer 1997; 71: 340–4
- Powell J, McConkey CC. The rising trend in oesophageal adenocarcinoma and gastric cardia. Eur J Cancer Prev 1992; 1: 265–9
- Hansson LE, Sparén P, Nyrén O. Increasing incidence in both histological types of esophageal carcinomas among men in Sweden. Int J Cancer 1993; 54: 402–7
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85: 1457–9
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Cardiovasc Surg 1978; 76: 643–54
- Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994; 220: 364–72
- Sutton DN, Wayman J, Griffin SM. Learning curve for oesophageal cancer surgery. Br J Surg 1999; 86: 282
- Miller JD, Jain MK, de Gara CJ, Morgan D, Urschel JD. Effect of surgical experience on results of esophagectomy for esophageal carcinoma. J Surg Oncol 1997; 65: 20–1
- Rouvelas I, Lindblad M, Zeng W, Viklund P, Ye W, Lagergren J. Impact of hospital volume on long-term survival after esophageal cancer surgery. Arch Surg 2007; 142: 113–7
- Nygaard K, Hagen S, Sand Hansen H, Hatlevoll R, Hultborn R, Jakobsen A, et al. Preoperative radiotherapy prolongs survival in operable esophageal carcinoma: A randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992; 16: 1104–10
- Mei W, Xian-Zhi G, Weibo Y, Guojun H, Liang-Jun W, Da-Wei Z. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: Report on 205 patients. Int J Radiat Oncol Biol Phys 1989; 16: 325–7
- Gignoux M, Roussel A, Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: Results of a study of the EORTC. World J Surg 1987; 11: 426–32
- Kelsen D. Chemotherapy of esophageal cancer. Semin Oncol 1984; 11: 159–68
- Herskovic A, Martz K, Al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992; 326: 1593–8
- Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335: 462–7
- Stockeld D, Tennvall J, Wagenius G, Albertsson M, Backman L, Brodin O, et al. A Swedish study of chemoradiation in squamous cell carcinoma of the esophagus. Acta Oncol 2001; 40: 566–73
- Lagergren J, Bergström R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999; 340: 825–31
- Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett's) esophagus. N Engl J Med 1985; 313: 857–8