Abstract
Background. Long-term survivors of childhood malignancy are a newly emergent patient group with a unique but wide range of survivorship issues. There are rising demands for long-term, medical follow-up and social support for this potentially vulnerable group. These demands stem from improving survival rates and the progressively increasing incidence of late physical, psychological and social sequelae. Case-studies and Discussion. The ideal method to facilitate this long-term follow-up is unclear, and faces the problems of health-care system limitations coupled with the paucity of outcomes-based research to guide evidence-based, clinical practice. We discuss how the Late Effects Clinic operates in our institution: optimising the involvement of the multi-disciplinary medical and allied health care teams to meet the physical and psychological needs of long-term survivors, and to assist with the social issues surrounding survivorship. This model involves a co-operative team approach, thereby alleviating the sole responsibility from general practitioners or individual physicians with a restricted realm of expertise. We present three case reports illustrating the value of a formal late effects follow-up programme, and demonstrating the integration of the Late Effects Clinic into medical practice.
Over the past decade, the increasing survival rates following treatment for paediatric malignancies have increased the focus on the need for long-term follow-up to address survivorship issues Citation[1–3]. Approximately two thirds of survivors of childhood cancer experience at least one late effect, while approximately one quarter experiences a late effect that is severe or life-threatening Citation[4], Citation[5]. The role of a late effects programme is to follow survivors through childhood, adolescence and into adulthood; to screen and manage the long-term physical and neuropsychological sequelae of the disease and previously delivered oncological therapies, as well as assisting with the social issues associated with long-term survivorship. These aspects are distinct from the short- and intermediate-term goals of oncology follow-up, which are primarily aimed at the early detection of disease relapse and the management of early, treatment-related complications. The following three cases highlight the value of a formal, multi-disciplinary, late effects programme in the long-term management of survivors of paediatric malignancies.
Case study 1
In 1971, at the age of 17, Miss AS was diagnosed with stage IIIB mixed cellularity Hodgkin lymphoma. Following staging laparotomy, she was treated with extended-field radiotherapy (mantle and para-aortic field) to 20 Gy, followed by a reduction in field size to boost the involved-field to a total dose of 32 Gy in 16 fractions at 5 fractions per week. In 1976, she developed a marginal recurrence of biopsy-proven Hodgkin lymphoma, presenting with right cervical lymphadenopathy. She received salvage chemotherapy with six cycles of mechlorethamine, vincristine, procarbazine and prednisolone (MOPP).
In 1999, routine examination revealed a palpable right-sided thyroid nodule, and she subsequently underwent a right hemi-thyroidectomy. Histopathology was reported as a 2 mm focus of papillary carcinoma. In view of the good prognosis, no further surgery or adjuvant radio-iodine therapy was recommended.
In 2001, Miss AS was referred to the Late Effects Clinic (LEC) by her haematologist. At this time, she was a 47 year old unmarried, nulliparous woman. Examination revealed mild skin changes consistent with previous radiotherapy, no goitre, no palpable breast masses, normal cardiologic and respiratory examinations, and no palpable lymphadenopathy. Endocrinology assessment revealed inadequate suppression of thyroid stimulating hormone, given her prior thyroid carcinoma, and her dose of thyroxine was increased. The patient also suffered chemotherapy-induced ovarian failure: hormone replacement therapy was discussed but she did not wish to proceed. A mild reduction in bone mineral density was found on screening. Cardiology assessment (including baseline electrocardiograph, echocardiogram, fasting lipid profile and blood pressure checks) was within normal limits.
In the LEC, the planned programme for Miss AS consisted of annual clinical assessment and regular screening investigations. Thyroid function was assessed annually, and two-yearly bone mineral densitometry was recommended. In view of the risk for second malignancies, annual full blood examination, chest x-ray and yearly bilateral mammography were recommended. As Miss AS was no longer receiving specific oncological follow-up for the previous thyroid malignancy, annual surveillance thyroid ultrasounds were also organized. Miss AS was also advised to receive annual influenza vaccination plus five-yearly pneumococcal and meningococcal vaccinations, in view of her previous splenectomy.
In 2003, screening mammography revealed abnormalities in the right breast suggestive of malignancy. She underwent right mastectomy, sentinel lymph node biopsy followed by right axillary dissection. The histopathology was reported as two synchronous ductal carcinomas in the right breast, 14 mm and 8 mm respectively, both grade three, with lymphovascular space invasion. Immunohistochemical staining for oestrogen and progesterone receptors were positive, and Her-2-neu was negative. Two of nine axillary lymph nodes were involved with metastatic carcinoma. Staging investigations were clear of distant metastatic disease. Adjuvantly, Miss AS received four cycles of adriamycin and cyclophosphamide, and subsequently commenced a planned five year course of tamoxifen.
In parallel with her breast cancer management and surveillance, Miss AS continued annual review in the LEC on the planned programme. In 2006, thyroid ultrasound revealed a 3.8×2.5mm hyper-echoic nodule in the residual left lobe of the thyroid, suspicious of recurrent papillary carcinoma. In addition, screening chest x-ray demonstrated new lung nodules, confirmed on CT scan. Biopsy of the lung nodules confirmed metastatic breast carcinoma. Further investigations also revealed bone metastases. She continues under the care of her medical oncologist, and is receiving systemic therapy for palliation of her metastatic breast cancer. As the thyroid nodule is small and asymptomatic, completion thyroidectomy is not currently planned given her overall circumstances.
Miss AS does not have a family history of malignancy to suggest a hereditary predisposition, and it is felt that the thyroid and breast malignancies are more likely to be radiation-induced secondary neoplasms.
Case study 2
Mr MR is a 31 year old man with a past history of precursor B acute lmphoblastic leukaemia (ALL), diagnosed at the age of 5 years and 10 months, in 1981. Cytogenetics were diploid and his baseline cranial spinal fluid (CSF) test was uninvolved. Induction chemotherapy was a combination of vincristine, cytosine arabinoside, cyclophosphamide, L-asparaginase and intrathecal methotrexate. He received prophylactic cranial irradiation, fractionated to a total dose of 18 Gy. Maintenance chemotherapy consisted of oral 6-mercaptopurine and methotrexate.
In early 1986, he was found to have first relapse in the bone marrow, with normal CSF. He underwent re-induction with vincristine, daunorubicin, prednisolone, L-asparaginase and intrathecal methotrexate. This was followed by consolidation systemic chemotherapy and intrathecal methotrexate, and then maintenance with oral 6-mercaptopurine and methotrexate.
In July 1986, he had a second relapse and was re-induced with vincristine, doxorubicin, dexamethasone, L-asparaginase. Consolidation chemotherapy consisted of cyclophosphamide 1g/m2, with cytosine arabinoside and 6-thioguanine. Maintenance treatment followed, with vincristine, cyclophosphamide, daunorubicin, 6-mercaptopurine and methotrexate.
A third relapse was diagnosed in 1989, detected in both bone marrow and CSF. Mr MR underwent re-induction with vincristine, daunorubicin, prednisolone, L-asparaginase, intrathecal methotrexate, followed by consolidation and maintenance chemotherapy. In March 1990, the patient underwent a sibling allograft. The preparative regimen was with busulphan, cytosine arabinoside and cyclophosphamide. He received cyclosporin and methotrexate for graft versus host disease (GVHD) prophylaxis, and engraftment occurred with no evidence of GVHD.
In 1991, Mr MR suffered a fourth relapse isolated to the central nervous system, presenting with bilateral lower motor neurone signs. He underwent re-induction with vincristine, daunorubicin, dexamethasone, L-asparaginase. At this time, his total cumulative anthracycline dose was 420 mg/m2. Following consolidation chemotherapy, the patient received craniospinal irradiation fractionated to a total dose of 42 Gy to his brain and 24 Gy to his spine. Oral maintenance chemotherapy was interrupted by an episode of Neisseria meningitides septicaemia, and was re-commenced after recovery to complete the full course. He remains in complete remission: now disease-free for 15 years after his last relapse.
Twelve years after his last relapse, at the age of 28, Mr MR was referred to the LEC. He had developed numerous treatment-induced morbidities, both physical and psychological.
Physical, treatment-induced morbidities were skeletal, cardiac and neurological. Prior to his referral to the LEC, Mr MR had sustained bilateral avascular necrosis of the femoral heads, requiring total hip joint replacements in 1993 and 1994. Echocardiogram in 1991 had revealed a fractional shortening of 40%, deteriorating to 29% in 1996 with left ventricular dilatation. After cardiology assessment in the LEC, the cardiologist recommended ongoing monitoring with annual examination, plus five yearly echocardiography, electrocardiography and annual fasting lipid profile. Audiometry testing was undertaken as part of the neurological assessment in the LEC, and this demonstrated mild, low-frequency, sensorineural hearing loss bilaterally.
The late effects programme for this patient also included regular screening investigations for second malignancies: annual full blood examination and urine cytology, two yearly thyroid ultrasound and five yearly CT scan from head to pelvis through the radiotherapy field. Endocrinology screening included yearly pituitary, thyroid, adrenal, and gonadal hormone levels, plus five yearly bone mineral density testing. Liver and renal function were tested yearly. Viral serology for Hepatitis-B, -C and HIV were also tested in view of numerous blood transfusions previously received. To date, these screening tests have all been consistently within normal limits.
His childhood and adolescent experiences had a significant impact on his psychological and social functioning in adulthood. Mr MR was suffering nightmares about his earlier treatment, and described symptoms of previously undiagnosed depression. He was also extremely socially isolated. His family background was complicated by an abusive father, and the breakdown of his parents’ marriage which coincided with his final relapse of ALL. Whilst he had initially benefited from the social interaction in a youth programme designed for children and adolescents affected by cancer, he had lost contact with his peers once he became too old for this programme, and Mr MR had steadily become more socially withdrawn.
From the LEC, Mr MR was referred to a psycho-oncologist and neuro-psychologist for assessment and intervention. He received orientated supportive expressive therapy and cognitive behavioural interventions for management of anxiety, anger and stress. Mr MR was also commenced on anti-depressant medication. Throughout, close liaison with his local doctor was important, particularly as Mr MR lived in a rural town distant from our centre.
Today, Mr MR continues his Late Effects programme. Communication with his local doctor remains integral to the optimal delivery of his care. The benefits of his intensive therapy with the psycho-oncologist are apparent, with improving self-esteem, widening social network, new employment and ambitions for promotion. He is also a keen participant in the social programme organised through the LEC for survivors of paediatric malignancy.
Case study 3
Ms RL, a 50 year old woman, had previously received orthovoltage radiotherapy to a strawberry naevus on the left anterior chest wall as an infant. Ms RL self-referred to the LEC due to her concerns about the risk of second malignancy following radiotherapy. Details of her previous radiotherapy were not obtainable. The patient's co-morbidities included multi-nodular goitre, dyslipidaemia and congenital mitral valve prolapse. She was a non-smoker with a family history of ischaemic heart disease. The patient was asymptomatic at presentation. On examination, the left anterior chest wall had an area of skin pallor, telangiectasia and atrophy, without underlying fibrosis, measuring 12 cm in diameter. A thyroid goitre was palpable. There were no suspicious palpable breast masses, skin lesions or regional lymphadenopathy. Clinically, the patient was euthyroid.
Screening investigations included a CT of the chest and neck, bilateral breast ultrasound and mammogram, and thyroid imaging. She was reviewed by a head and neck surgeon linked to the LEC. In view of the risk of radiation-induced second malignancy of the thyroid gland, potentially masked by the multinodular goitre, total thyroidectomy was recommended. The histopathology revealed a 6 mm focus of papillary carcinoma. Due to the good prognostic disease, adjuvant radio-iodine ablation therapy was not recommended. She continues on a surveillance policy with annual thyroid ultrasound.
The late effects programme for this patient comprises annual bilateral screening mammograms and skin checks for second malignancies. The radiation dose to the heart was most likely small, but unconfirmed. After discussion, it was decided that baseline echocardiogram and electrocardiogram would be recommended, however continued cardiac surveillance was not required. Referral to the LEC also presented an opportunity for primary health care education, including dietary advice to minimise the risk of ischaemic heart disease.
Discussion
The need for long-term, multidisciplinary follow-up is pertinent for this vulnerable population of cancer survivors. Retrospective cohort studies have demonstrated that paediatric cancer survivors have elevated risks of chronic, multi-system, physical effects Citation[5]. The risk of treatment-induced second malignancies is difficult to quantify: standardized incidence ratios range from 5–15% in retrospective series Citation[6–9]. Psychological and psychiatric problems are also recognised risks Citation[10], with reports suggesting that poor self-esteem is manifest in approximately 20% of survivors Citation[11], and approximately 13% incidence of clinically significant, post-traumatic stress disorder Citation[12], Citation[13]. Social sequelae in survivors of childhood malignancies include difficulties forming friendships Citation[14], schooling problems with 26% requiring special education assistance Citation[15], and an unemployment rate twice that of controls Citation[16].
The requirement for long-term, medical follow-up for survivors of childhood malignancy is a growing problem. Population-based studies have demonstrated a substantial improvement in survival over the past 40 years, and modern data suggests that 70% of children with malignancy can expect to survive five years or more Citation[17]. With time, the risk of second malignancy increases and progressive deterioration in organ function results in excess morbidity and mortality rates Citation[5], Citation[18], Citation[19]. The Childhood Cancer Survivor Study of 10 397 survivors, aged 18–48 years, reported that at 30 years the cumulative rate of chronic morbidity in survivors of paediatric malignancies was 73.4%, and the cumulative rate of severe or life-threatening conditions reached 42.2% Citation[5]. Therefore, life-long follow-up is particularly important in view of the long natural history for the development of late sequelae of cytotoxic therapies. This latency period allows for screening and early intervention, and facilitates minimisation of contributing risk factors by education and primary prevention Citation[2].
The optimal method for long-term follow-up is unclear. Most oncologists recommend life-long, risk-adjusted care, with access to a multi-disciplinary team Citation[20], Citation[21]. However, key limitations to the provision of this follow-up are difficult to overcome Citation[22–24]. At the level of the health-care system, insufficient specialist expertise, inadequate funding to sustain follow-up programs and low capacity to accommodate the growing number of survivors, are serious barriers to the provision of long-term follow-up. At the patient level, limitations include inadequate appreciation of the individuals’ health risks, lack of knowledge regarding preventative health care measures, and poor memory recall of previous childhood cancer treatments, compounded by increasingly transitory lifestyles or losses to medical follow-up. And at the level of the general practitioner, the lack of knowledge of previous oncological therapies and the relevant risks of late toxicity, limitations in communication with multi-disciplinary specialists, plus the infrequency of childhood cancer survivors in community practice, makes long-term follow-up a difficult task.
Furthermore, the most effective surveillance programme is also unclear Citation[24]. Ideally, this should follow an evidence-based and risk-adjusted protocol to avoid excessive and cost-ineffective tests. However, the reality of clinical practice is made problematic by the paucity of available evidence examining the optimal frequencies or choice of modalities for screening and follow-up in this population group. To assist health care providers, the Children's Oncology Group has published exposure-based guidelines for long term screening and follow-up for childhood cancer survivors Citation[25].
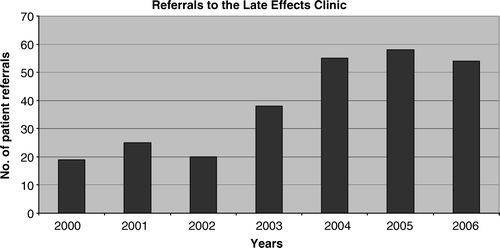
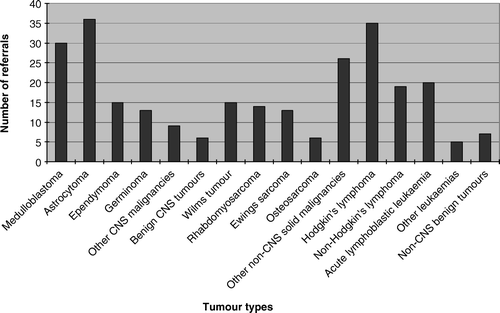
A survey of members of the Children's Cancer Group and Paediatric Oncology Group concluded that few programmes focused on the long-term health care needs of adult survivors of paediatric malignancies, and that the majority of programmes operated in paediatric institutions and lacked the input of adult-orientated medical practitioners Citation[26]. At the Peter MacCallum Cancer Centre, the LEC is established at a primarily adult-based hospital, and is fortunate to receive the support of paediatric oncology departments around the state who refer their patients for long-term follow-up of late effects. The LEC was established in 2000, and since that time, 269 patients have been referred for long-term follow-up (), with a wide spectrum of underlying diseases (). Of the 269 referrals, 49.4% came from children's hospitals, 38.3% from adult hospitals, 6.3% were referred from general practitioners, 4.1% were self-referrals, and 1.9% came from other sources.
At the Peter MacCallum Cancer Centre, the LEC advocates regular life-long follow-up for all paediatric malignancies, from the time of diagnosis through to adolescence and adulthood. In general, patients are accepted to the LEC at least 5 years after completion of therapy: a time which traditionally has been associated with reduced access to resources and supports, for patients in remission. The charter of the LEC is to address physiological and psychological treatment-induced sequelae and disease-related effects. It offers an individualised, risk-based strategy that considers primary functional deficits from the malignant process, previous cancer treatments, genetic predisposition, co-morbidities, neuro-cognitive function and social circumstances. This programme is designed to operate in parallel with the primary managing specialists, who are responsible for the ongoing surveillance for disease recurrence, if relevant. In our practice, the ongoing management of this vulnerable patient population continues long after the cure of their disease.
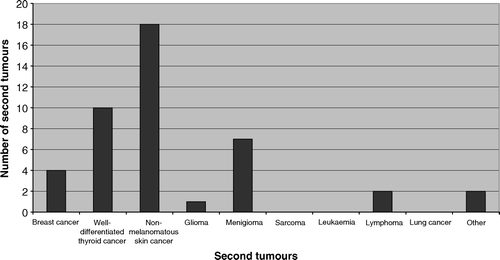
The first case study described here illustrates the long-term risk of second malignancy as a significant survivorship issue. Second malignancy has been found to be the leading cause of death in 15+ year survivors of childhood malignancies Citation[18]. Since the opening of the LEC at the Peter MacCallum Cancer Centre, 44 patients have been diagnosed with second neoplasms, with a median latency period of 21 (7–50) years. Fortunately, the majority of these second neoplasms are not generally considered to be life-threatening, however, there is a clinically significant proportion with more sinister pathologies (). The first case report also highlights the importance of multi-disciplinary involvement, and the benefit of a positive working relationship between the Late Effects team and the primary treating physician. The second case study was selected to demonstrate the psychological issues that can trouble survivors of childhood malignancy. There is growing evidence of long-term psychological and neuro-cognitive dysfunction in survivors, suggesting that these effects have been previously under-estimated. Unfortunately, in the wider community, access to appropriate services for identification and intervention is often restricted Citation[5]. As in this case, close communication with the community-based, primary care physician is advantageous for the optimal care of psycho-social and active health issues. Lastly, the third case study demonstrates that radiation therapy for benign conditions can lead to serious late effects: these patients also warrant referral to a dedicated LEC for long-term follow-up.
It is well recognised that it is difficult for individual health care providers to co-ordinate the appropriate long term follow-up for a heterogenous group of patients Citation[21]. Our purpose-designed LEC, attended by dedicated multi-disciplinary specialists, overcomes this problem. The consultative team in the LEC includes radiation oncologists, haematologists, medical oncologists, endocrinologists, a cardiologist, a neurologist, a dedicated nurse coordinator and a social worker. Prior to each clinic, all scheduled patients are discussed in a multidisciplinary meeting with regard to anticipated health risks, relevant past issues and the appropriate screening investigations. The clinic is patient-orientated: all relevant practitioners review the patient on the same day, and the patients remain in the one location throughout and therefore are not required to move from room to room for consultations with the relevant practitioners. For the patient, this arrangement is less daunting; and for the specialists, it facilitates a co-ordinated, multidisciplinary assessment. Purposefully linked to the LEC are a head and neck surgeon, respiratory physician, ophthalmologist, reproductive medicine specialist, psycho-oncologist, neuro-psychologist, occupational therapist, physiotherapist and dietician; although these specialists are not present for the consultative clinics, they offer a specialised service to the patients of the LEC. Regular screening is conducted for treatment-induced and disease-related adverse effects including neuro-cognitive effects, endocrine abnormalities, visceral dysfunction, neurological sequelae, growth effects and second malignancy. Neuro-psychological testing identifies specific cognitive impairments and capabilities, and helps in directing patients towards future employment or training choices. Psycho-oncologists and social workers work to assist the integration of the patient in the community. Social activities and regular newsletters are purpose-driven to encourage patients who feel socially isolated by their experiences or late effects.
An additional benefit of our LEC is the construction of a database of survivors of childhood malignancy. Maintenance of this database allows for data collection analyses and serves as an educational platform regarding late toxicities and patient needs. The database also facilitates tracking survivors who are receiving community-based follow-up, or who may otherwise be at risk of becoming “lost to follow-up”.
Conclusion
For survivors of childhood malignancy, enrolment in a LEC programme is as important as long-term oncological surveillance. The LEC at Peter MacCallum Cancer Centre offers a life-long commitment to the physical and psychological well-being of this population. We advocate a multidisciplinary approach for optimal patient care in terms of education, screening, intervention and primary preventative health care.
References
- Hollen PJ, Hobbie WL. Establishing comprehensive specialty follow-up clinics for long-term survivors of cancer. Providing systematic physiological and psychosocial support. Support Care Cancer 1995; 3: 40–4
- Hinkle AS, Proukou C, French CA, Kozlowski AM, Constine LS, Lipsitz SR, et al. A clinic-based, comprehensive care model for studying late effects in long-term survivors of pediatric illnesses. Pediatrics 2004; 113(4 Suppl)1141–5
- Hudson MM. A model for care across the cancer continuum. Cancer 2005; 104(11 Suppl)2638–42
- Bhatia S, Landier W. Evaluating survivors of pediatric cancer. Cancer J 2005; 11: 340–54
- Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355: 1572–82
- MacArthur AC, Spinelli JJ, Rogers PC, Goddard KJ, Phillips N, McBride ML. Risk of a second malignant neoplasm among 5-year survivors of cancer in childhood and adolescence in British Columbia, Canada. Pediatr Blood Cancer 2007; 48: 453–9
- Jenkinson HC, Hawkins MM, Stiller CA, Winter DL, Marsden HB, Stevens MC. Long-term population-based risks of second malignant neoplasms after childhood cancer in Britain. Br J Cancer 2004; 91: 1905–10
- Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood cancer survivor study. J Natl Cancer Inst 2001; 93: 618–29
- de Vathaire F, Schweisguth O, Rodary C, Francois P, Sarrazin D, Oberlin O, et al. Long-term risk of second malignant neoplasm after a cancer in childhood. Br J Cancer 1989; 59: 448–52
- Peterson CC, Drotar D. Family impact of neurodevelopmental late effects in survivors of pediatric cancer: Review of research, clinical evidence, and future directions. Clin Child Psychol Psychiatry 2006; 11: 349–66
- Seitzman RL, Glover DA, Meadows AT, Mills JL, Nicholson HS, Robison LL, et al. Self-concept in adult survivors of childhood acute lymphoblastic leukemia: A cooperative Children's Cancer Group and National Institutes of Health study. Pediatr Blood Cancer 2004; 42: 230–40
- Langeveld NE, Grootenhuis MA, Voute PA, de Haan RJ. Posttraumatic stress symptoms in adult survivors of childhood cancer. Pediatr Blood Cancer 2004; 42: 604–10
- Lee YL, Santacroce SJ. Posttraumatic stress in long-term young adult survivors of childhood cancer: A questionnaire survey. Int J Nurs Stud 2006.
- Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer 2005; 104: 1751–60
- Mitby PA, Robison LL, Whitton JA, Zevon MA, Gibbs IC, Tersak JM, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2003; 97: 1115–26
- de Boer AG, Verbeek JH, van Dijk FJ. Adult survivors of childhood cancer and unemployment: A metaanalysis. Cancer 2006; 107: 1–11
- Curry HL, Parkes SE, Powell JE, Mann JR. Caring for survivors of childhood cancers: The size of the problem. Eur J Cancer 2006; 42: 501–8
- Lawless SC, Verma P, Green DM, Mahoney MC. Mortality experiences among 15+ year survivors of childhood and adolescent cancers. Pediatr Blood Cancer 2007; 48: 333–8
- Green DM, Hyland A, Chung CS, Zevon MA, Hall BC. Cancer and cardiac mortality among 15-year survivors of cancer diagnosed during childhood or adolescence. J Clin Oncol 1999; 17: 3207–15
- Alvarez JA, Scully RE, Miller TL, Armstrong FD, Constine LS, Friedman DL, et al. Long-term effects of treatments for childhood cancers. Curr Opin Pediatr 2007; 19: 23–31
- Friedman DL, Freyer DR, Levitt GA. Models of care for survivors of childhood cancer. Pediatr Blood Cancer 2006; 46: 159–68
- Aziz NM, Oeffinger KC, Brooks S, Turoff AJ. Comprehensive long-term follow-up programs for pediatric cancer survivors. Cancer 2006; 107: 841–8
- Skinner R, Wallace WH, Levitt GA. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol 2006; 7: 489–98
- Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: Education, surveillance, and screening. Pediatr Blood Cancer 2006; 46: 149–58
- Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group long-term follow-up guidelines from the Children's Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 2004; 22: 4979–90
- Oeffinger KC, Eshelman DA, Tomlinson GE, Buchanan GR. Programs for adult survivors of childhood cancer. J Clin Oncol 1998; 16: 2864–7