Abstract
Purpose. To investigate effects of blood transfusion, with/without leucocyte depletion, on duration of hospital stay, need for respiratory support, mortality and long-term survival after curative surgery for colorectal cancer. Methods. The trial was a prospective, randomised, multicenter study. Six hundred and forty two patients with colorectal cancer were included. Blood transfusion was given when needed during and/or after operation, randomised to packed red blood cells (RBC) or leucocyte-depleted red blood cells (LDB) using leucocyte filtration. Assisted ventilation in ICU, hospital stay, malignant and non-malignant specific mortality and overall survival were outcome measures. Results. The RBC group had higher need for assisted ventilation post-operatively (8.1% vs. 3.6%) and significantly higher proportion of patients with prolonged (>20 days) hospital stay. After median follow-up time of 99.5 months there was no significant difference in mortality or long-term survival between the groups. The median cumulative survival time of 55 months in LDB vs. 36 months in RBC group did not reach significance level. Non-transfused patients had a significantly lower proportion of prolonged hospital stay, and significantly increased survival, compared to transfused patients. Conclusion. Leucocyte depleted transfusions improved the postoperative course following surgery for colorectal cancer, compared with packed red blood cell transfusions.
The immunomodulating effects of blood transfusion were first reported in connection with kidney transplantation resulting in improved graft survival if blood transfusion was given prior to transplantation Citation[1]. Increased risk of postoperative infectious complications Citation[2] and fewer acute episodes of Crohn's disease have also been reported after blood transfusion Citation[3]. Several retrospective studies have indicated decreased long-term survival in patients receiving transfusion of blood products in connection with cancer surgery Citation[2], Citation[4], Citation[5]. Immunosuppression through transfused allogeneic leucocytes, which can influence the activity of endogenous NK-cells and change the ratio of T-helper/T-suppressor cells, has been suggested as a possible mechanism Citation[6]. Jensen et al. reported lower incidence of postoperative infections after colorectal surgery in patients who received leucocyte-depleted blood Citation[7]. No difference in cancer-free long-term prognosis has been detected after colorectal cancer surgery in previously reported prospective randomised studies comparing transfusions of autologous or leucocyte-depleted blood products with standard allogeneic packed red cell products Citation[8], Citation[9].
The hypothesis under test was that leucocytes in transfusion products change the immunologic response to cancer surgery which in turn may decrease the long-term survival after curative surgery.
The primary endpoint in this randomised trial was to compare malignant and non-malignant specific mortality and long-term overall survival of patients having received blood transfusion with or without leucocyte depletion in relation to curative surgery for colorectal cancer and to compare these two randomised groups to non-transfused controls.
Secondary endpoints were to compare the need of assisted ventilation in the ICU and the duration of hospital stay between the patients who received leucocyte depleted blood products with those who received standard buffy-coat-poor blood products and with patients who did not receive blood products in relation to curative surgery for colorectal cancer.
Patients and methods
The trial was approved by the Local Ethical Committee. Informed consent was received from all patients.
Starting in 1989 a total of 913 patients with gastrointestinal malignant tumours or kidney cancer were included into the trial until 1995 when inclusion was stopped. Seven hospitals in Western Sweden included patients who were to receive either leucocyte-depleted blood products (LDB) or ordinary buffy-coat depleted red blood concentrates and plasma (RBC) in case they needed transfusion during and after surgery. Patients who had received blood products outside of this protocol but within 6 days of operation were excluded from the trial. Patients who received blood products within 6 days of operations could be included if randomised in accordance with the protocol.
Randomisation was performed according to even or uneven date of birth. Patients with even date of birth were allocated to LDB group and those with uneven date of birth were allocated to RBC. The randomisation was not stratified for participating centre, pre- or postoperative chemo – or radiotherapy. At the time of inclusion there was no active program for systematic adjuvant treatment of solid gastrointestinal malignant tumours in the participating hospitals.
The transfusion period was defined in the protocol as “six days before surgery to 30 days after surgery”. In the preoperative period the need for transfusion was decided by the attending specialist regardless of whether this was a gastroenterologist or a surgeon. The decision was made according to the clinical situation and the transfusion policy in each department. The need for transfusion during the operation and the immediate post-operative period (recovery room or ICU) was decided by the anaesthetist in attendance and was based on an individual assessment of each patient and in accordance with the transfusion policy of the department. During the postoperative period in the surgical ward the decision to transfuse was made by the attending surgeon. In each participating hospital a discussion between the principle investigator (EH) and the local anaesthetists and surgeons on the transfusion policy took place before the start of inclusion into the trial. This discussion was repeated at general meetings with representatives from all the participating hospitals every 6–12 months throughout the inclusion period.
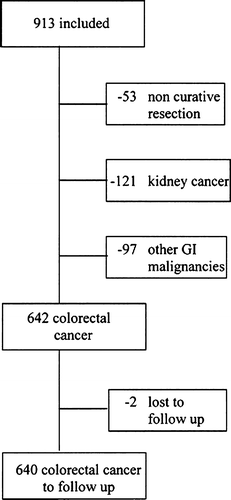
According to the inclusion criteria only patients with a potentially curative surgical procedure were included in the trial. In 53 patients it was either not possible to perform a radical surgical excision or distant metastases were discovered per-operatively. These patients were subsequently excluded from the study in accordance with the exclusion criteria in the protocol. Of the remaining 860 patients in the study, 642 patients had colorectal cancer, 121 kidney cancer and 97 patients were divided into six different categories of gastrointestinal malignancies (). For the sake of uniformity we chose the largest subgroup, colorectal cancer, for follow-up and further analysis. The Register of Inhabitants for Sweden was used to control that all patients were accounted for. Two patients were not traceable in this register and thus lost to follow-up (). The follow-up was exclusively through the National Death Registry. This was searched in order to identify the patients who were included in the trial and who had died up to January 1, 2001. Of the 642 patients with colorectal cancer 298 patients (46%) were actually transfused. One hundred and thirty seven patients received LDB and 161 patients received RBC. The remaining 344 patients who were included into the trial without transfusions served as a non-transfused patient group (NTP) within the trial.
Figure 1. Flow chart of the trial population, reasons for exclusion and resulting population studied.
Leucocyte depletion was accomplished by a flat-bed polyester filter, Sepacell R-500A (VingMed AB). This is a highly effective filter which results in >3 log 10 leucocyte reduction Citation[10] and has been reported to be effective for two units of whole blood or red cells Citation[11]. Each filter was used for a maximum of two units of buffy-coat removed red cell concentrates or two units of plasma. The filtration was performed “bedside”. For patients randomised to LDB this was marked in the patient file by stating which group the patient had been randomised to and by a colour code of the inclusion statement (green for LDB and pink for RBC). When a need for transfusion occurred the filter was connected to the unit of blood or plasma before connecting to the patient in the LDB group whereas the unit was directly connected to the patient in the RBC group. The service for blood transfusion was not in any of the hospitals aware of whether or not patients, for whom they cross-matched blood products, were included into the trial. In the LDB group 56 patients (41%) received one or more unfiltered blood or plasma units due to technical failure, negligence or the demand for speedy transfusions (). These patients were handled according to “intention to treat” when data was collected and analysed.
Table I. Filtration mistakes.
Data on number of days in need of respiratory support, number of days in the ICU and length of hospital stay were registered prospectively in accordance with the protocol. Before analysis of data a limit of 20 days in hospital was chosen as the upper limit for what should be regarded as “normal” length of hospital stay. The protocol for the trial did not include registration of infectious complications, as the interesting link between blood transfusions and infectious complications had not been suggested in 1988–1989 when the protocol was finalised and inclusions started.
Surgical procedures were standard open colon or rectal resections which in all participating hospitals at the time were on the following principles: “high tie”, “no touch” and traditional curative resection, i.e. right hemicolectomy, left hemicolectomy, sigmoid resection, anterior resection or abdominal-perineal resection. Total mesorectal excision had not been introduced in the participating hospitals at the time of the inclusions.
Statistical methods
Power calculation
Five year survival was the primary endpoint in the trial, whereas length of hospital stay and need of respiratory assistance were secondary endpoints. If the expected 5-year survival in the group with the worse outcome was 55% and the difference in survival between the two groups was 15%, i.e. increased 5-year survival in the other group to at least 70%, the power was 80% at a significance level of 0.05 (two-tailed) for comparison of the two groups with a minimum of 170 patients in each group.
The distribution of age and the number of received units of blood product within the groups were calculated by a standard computer statistical program (StatView, Abacus Concepts Inc.). Fisher's exact test was used to assess the significance of difference between subgroups concerning length of hospital stay and the need of respiratory support. Survival curves were calculated according to Kaplan-Meier followed by the log-rank (Mantel-Cox) test to compare differences between groups. Throughout, p-values below 0.05 were regarded as statistically significant, with two-tailed test.
Multivariate analyses were performed by regression analysis according to Cox proportional hazards or by a Weibul model.
Results
The median age of the patients at the time of entry into the trial was 72 years (range 34–91), with variations between groups (p < 0.01) (). The median hospital stay for all patients was 13 days (range 5–144) with 16 days for the transfused patients and 11 days for the NTP group (p < 0.01) (). Patients with rectal cancer had a significantly longer hospital stay (p < 0.001) compared to colon cancer patients, which was expected. This was not related to number of or type of transfusion. Length of hospital stay was not related to Dukes’ stage.
Table II. Demographics.
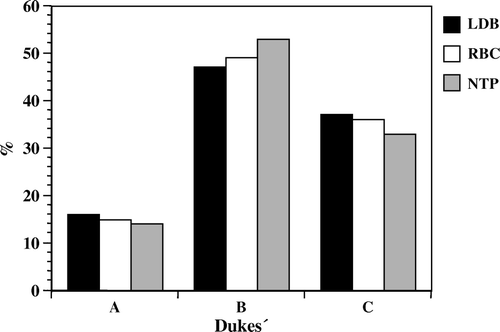
The mean number of blood and plasma units in LDB and RBC did not differ (NS) (). The number of rectal cancers was 250 (40%) and differed in relative proportion between the two transfused groups (LDB 54%, RBC 43%) (p < 0.05). The NTP group had a relatively smaller number of rectal cancers (31%) compared to the other two groups (p < 0.05). Staging according to Dukes’ classification in the total material was as follows: Dukes’ A–15%, Dukes’ B–51%, Dukes’ C–34%. The relative distribution of cancers according to Dukes'classification was similar in all three groups without significant differences (NS) ().
Figure 2. Relative distribution of patients according to Dukes’ classification, in the study groups. LDB represents patients transfused with leucocyte depleted blood products, RBC patients transfused with standard un-filtered blood products and NTP, non-transfused patients.
Need for respiratory support in ICU
Of the total number of 642 patients, respiratory support (RS) in the ICU was needed for 22 patients for one or several days. Six hundred and seventeen patients had no need for respiratory support (NRS) and in three patients information of ICU status was missing. There was a significant difference concerning the need for respiratory support between transfused patients (18 of 298) and non-transfused controls (4 of 344). In the transfused patients respiratory support was given to 13 in the RBC (n = 161) and five in the LDB group (n = 137) respectively. The difference between LDB and RBC was not statistically significant (hazard ratio (HR): 2.2;95% confidence interval (CI) 0.8 to 6.1; p = 0.144. The time with respiratory assistance was not a simple variable and was not explained by age, number of transfusions, type of cancer or whether or not leucocyte depleted transfusions had been given.
The median length of hospital stay in the RS group was 20 days (6–144) and for NRS patients 13 days (5–125).
Prolonged hospital stay
The definition of prolonged hospital stay was set to more than 20 days in hospital and accounted for 118 patients who were distributed as follows: 32 in LDB, 55 in RBC and 31 patients in NTP. The two groups with transfused patients (LDB and RBC) had a significantly (HR 3.2;95% CI 2.2 to 4.7; p < 0.001) higher percentage of patients with prolonged hospital stay compared to the group with non-transfused patients (NTP). The RBC group had also a significantly (HR 1.5;95% CI 1.0 to 2.1; p = 0.042) higher percentage of patients with prolonged hospital stay than the LDB group. The median length of stay for those with hospital stay >20 days, differed significantly between NTP and LDB (p < 0.01) (). Median age and mean number of received blood and plasma units did not differ between the LDB group and the RBC group ().
Table III. Patients with extended (>20 days) hospital stay.
Survival
After a median follow-up time of 99.5 months (range 62–129) 295 patients had died, of whom 180 (61%) were deceased of causes directly related to colorectal cancer disease. In 106 patients the cause of death was not from colorectal malignancy and in nine patients the direct cause of death could not be established. The median time to death postoperatively was 33 months (range 0–111).
Surgery related mortality (within 1 month) was reported in three patients, 0.5%. The time to recurrence of the tumour was not related to number of transfusions.
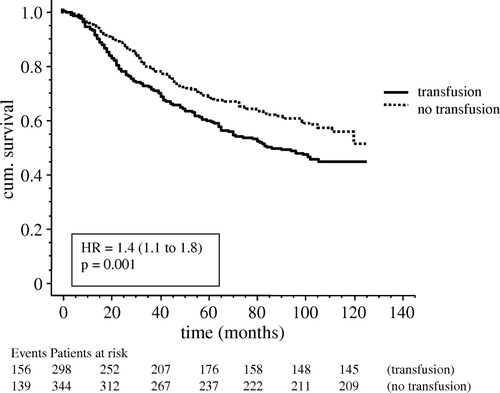
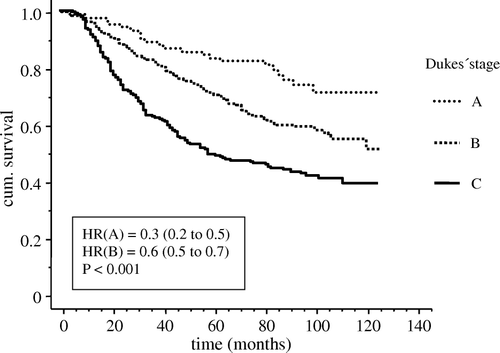
There was no difference in overall survival between patients transfused with LDB and RBC (). However, non-transfused patients had significantly (p = 0.017) longer survival compared to patients who received blood transfusion of any kind (). Overall 5-year survival according to Dukes’ stage showed significant difference (p < 0.001) between patients with Dukes’ A (78%), B (62%) and C (40%) stage, as expected (). There was no difference in survival between patients with colon cancer and patients with rectal cancer.
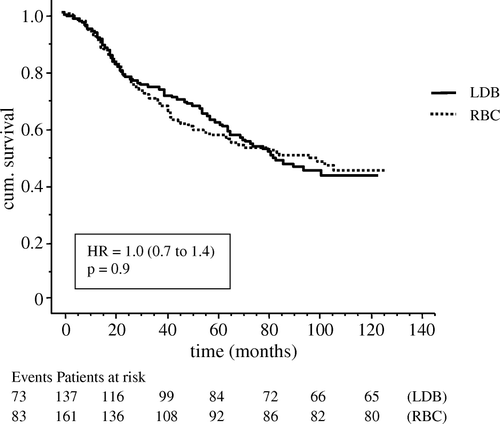
Figure 3. Survival curves for 298 patients who received blood transfusion with leucocyte depleted (LDB) or un-filtered (RBC) blood products. Differences were assessed by the logrank test and expressed as hazard ratio (HR) with 95% CI.
Malignant and non-malignant mortality
During follow-up 42 patients in the LDB group and 51 patients in the RBC group died of colorectal cancer. There were no significant differences in number of deaths or in survival time between the two transfused groups. There was no significant difference in death from non-malignant causes between the two transfused groups (30 and 29 patients in LDB and RBC respectively) during the follow-up period. The difference in median cumulative survival time, 55 months in LDB and 36 months in RBC (p = 0.05) did not quite reach significance level.
Discussion
In this trial we found no improvement in overall survival by leucocyte depletion of blood products after 6 years of follow-up of patients who had been treated by radical surgery for colorectal cancer. This is in agreement with most other studies where comparisons have been made between autologous and allogeneic transfused blood products, or between leucocyte diminished blood products and ordinary packed red blood cell concentrates and plasma Citation[8], Citation[9], Citation[12]. Our study also confirms the findings of previous studies, of both retrospective and prospective nature, that patients who received blood transfusion of any kind had a worse long-term prognosis compared with non-transfused patients Citation[5], Citation[7], Citation[8]. Mynster et al. reported a significantly increased risk of death in patients who developed infectious complications after transfusion in connection with colorectal cancer surgery Citation[13].
No prospective registration of infectious complications was included when the protocol in our trial was decided upon. In hindsight this was unfortunate, but at that time the link between infectious complications and transfusions had not been suggested. Other authors have reported that blood transfusion had an impact not only on post-operative infectious complications Citation[14] but also on morbidity in inflammatory bowel disease Citation[3], risk of habitual abortion and graft survival after kidney transplantation Citation[1], Citation[15]. In a recent meta analysis of eight randomised studies the authors concluded that patients transfused with leucocyte reduced blood might benefit by a reduction of postoperative infectious complications Citation[16]. It is therefore likely, that transfusion of blood products as such can alter or modulate pathophysiological responses to disease and trauma under certain circumstances.
Previous retrospective studies, reporting decreased long-term survival and shorter time-interval to tumour recurrence, led to the assumption of direct negative effects of blood transfusions on patient prognosis after surgery for malignant disease Citation[4]. The most commonly proposed mechanism has been immunosuppression through transfused allogeneic leucocytes Citation[6].
After a median follow-up time of 8 years it is not likely that a longer follow-up time would reveal any significant difference in cancer related mortality. The number of patients in each of the transfused study groups should be sufficient to detect a difference in overall 5 year survival exceeding 15%, according to the power calculation.
In some studies the risk for infectious complications was reported to be higher with increasing number of allogeneic transfusions Citation[17] in connection with surgery for gastric cancer and colon injuries. Further Triulzi et al. reported on increasing risk for postoperative infectious complications in spinal surgery with increasing number of allogeneic transfusions Citation[18]. Some studies have suggested a decreased long-term survival in relation to increasing amounts of transfused blood products Citation[19] or a “dose-effect” relationship between number of units transfused and recurrence Citation[20], whereas a prospective study in 468 patients with rectosigmoid/rectal cancers found that well-established prognostic factors accounted for the worsened prognosis in transfused patients, not transfusion as such Citation[21].
The high frequency (41%) of patients in our trial, in whom one or more units of blood or plasma was unfiltered even though the patients had been randomised to filter group, may be regarded as a flaw in the trial. The results have been analysed according to “intention to treat”. These missed filtrations may thus have influenced short- and long-term results in the filter group. We cannot completely exclude a negative influence of packed red cells on survival compared to leucocyte depleted red cells, as the power to detect such a difference was diminished by the missed filtrations. One could make the assumption that the transfusion-effect could be explained by selection. The reasoning would then be that larger or locally more advanced tumours, as well as preoperatively anaemic patients, would be selection criteria for transfusion and that the result would reflect such pre-existing determinants and not a detrimental effect of the blood transfusion itself. The explanation to the shorter overall survival among transfused patients compared with non-transfused patients, was not further clarified by multivariate analyses.
In recent years clinical trials have shown a higher risk of infectious, postoperative complications after transfusion of unfiltered compared to filtered blood components or after autologous blood transfusion as compared with homologous transfusion Citation[7], Citation[8], Citation[12]. On the other hand Fung et al. in a prospective case control study found no difference in postoperative infectious complications but shorter length of hospital stay when leucoreduction was used in connection with open heart surgery Citation[22]. In a leucoreduction program fewer episode of fever and less need of antibiotics were found post-transfusion when leucoreduction had been implemented as compared to the period before that Citation[23]. Our findings, that the LDB group had significantly fewer patients with a length of hospital stay > 20 days and fewer patients in need of assisted ventilation as compared to those transfused without filtration, could not be explained. Multivariate analyses did not explain this observation, accounting for age or colon versus rectal surgery. Without systematic registration of infectious complications, a relationship between postoperative infections and ICU care or need for respiratory support in the RBC group remains speculative. In coronary by-pass surgery and after colorectal cancer resection increased length of stay in the ICU and hospital has been reported after allogeneic blood transfusion Citation[24]. After both types of surgery there was a positive relation between amount of blood transfused and length of stay Citation[24].
Patients with immunosuppressive treatment after transplantation have increased risk of developing certain tumours such as lymphomas of the central nervous system and Kaposi's sarcoma compared to average risk in the population Citation[25]. This led to the hypothesis of increased risk for metachronous cancer after blood transfusions Citation[26]. Cerhan et al. reported a slightly higher incidence of certain malignant lymphomas and kidney cancer after transfusion Citation[26]. We found no increased risk of tumour development or worse prognosis in existing tumour disease in a study where we retrospectively followed two cohorts of women for 20–30 years after blood transfusion received at the time of child birth Citation[27].
The results in this trial support results in earlier reports that important consequences on post-operative complication rate and treatment cost-effectiveness occur following allogeneic blood transfusion and leucocyte filtration Citation[7], Citation[24]. However our conclusion that infectious complications could be one reason for extended hospital stay in patients who received red blood concentrates without filtration remains suggestive.
In conclusion patients who were not transfused at the time of radical surgery for colorectal cancer had a better prognosis compared to transfused patients. Blood transfusion with leucocyte containing products at the time of surgery for colorectal cancer did not influence patient survival, in a 5–6 year perspective. However the risk for postoperative complications decreased by the use of filtered blood products, when transfusion was needed. Thus, when blood transfusions are needed a regular use of leucocyte-filtration of blood products in patients undergoing surgery for colorectal cancer may be recommended.
Acknowledgements
The authors wish to thank Mrs Kristina Gustafsson and Harriet Törnqvist for secretarial and research assistance. The following hospitals took part in the trial and in patient accrual: Uddevalla hospital, Kungälv hospital, Alingsås hospital, Lidköping hospital, Mölndal hospital and Borås hospital. VingMed AB are gratefully acknowledged for supporting the trial by offering the filters at a non-profit price. This work was supported by grants from The Sahlgrenska University Hospital Foundation for Cancer Research and Development, Research projects financed by grants from the government under the LUA agreement, The Assar Gabrielson Foundation and The Jubileumskliniken Foundation.
References
- Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc 1973; 5: 253–9
- Tartter PI. Blood transfusion and infectious complications following colorectal cancer surgery. Br J Surg 1988; 75: 789–92
- Williams JG, Hughes LE. Effect of perioperative blood transfusion on recurrence of Crohn's disease. Lancet 1989; 2: 131–3
- Parrott NR, Lennard TW, Taylor RM, Proud G, Shenton BK, Johnston ID. Effect of perioperative blood transfusion on recurrence of colorectal cancer. Br J Surg 1986; 73: 970–3
- Jahnson S, Andersson M. Adverse effects of perioperative blood transfusion in patients with colorectal cancer. Eur J Surg 1992; 158: 419–25
- Heiss MM, Fasol-Merten K, Allgayer H, Strohlein MA, Tarabichi A, Wallner S. Influence of autologous blood transfusion on natural killer and lymphokine-activated killer cell activities in cancer surgery. Vox Sang 1997; 73: 237–45
- Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet 1996; 348: 841–5
- Houbiers JG, Brand A, van de Watering LM, Hermans J, Verwey PJ, Bijnen AB. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet 1994; 344: 573–8
- Jensen LS, Puho E, Pedersen L, Mortensen FV, Sorensen HT. Long-term survival after colorectal surgery associated with buffy-coat-poor and leucocyte-depleted blood transfusion: A follow-up study. Lancet 2005; 365: 681–2
- Masse M, Andreu G, Angue M, Babault C, Beaujean F, Bidet ML. A multicentre study on the efficiency of white cell reduction by filtration of red cells. Transfusion 1991; 31: 792–7
- Sirchia G, Rebulla P, Parravicini A, Carnelli V, Gianotti GA, Bertolini F. Leucocyte depletion of red cell units at the bedside by transfusion through a new filter. Transfusion 1987; 27: 402–5
- Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet 1993; 342: 1328–33
- Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg 2000; 87: 1553–62
- Braga M, Vignali A, Radaelli G, Gianotti L, Di Carlo V. Association between perioperative blood transfusion and postoperative infection in patients having elective operations for gastrointestinal cancer. Eur J Surg 1992; 158: 531–6
- Frisk B, Berglin E, Blohmé I, Persson H, Sandberg L, Wedel N. Clinical experience of blood transfusion in renal transplantation. Scand J Urol Nephrol Suppl 1984; 88: 1–69
- Fergusson D, Khanna MP, Tinmouth A, Hébert PC. Transfusion of leukoreduced red blood cells may decrease postoperative infections: Two meta-analyses of randomized controlled trials. Can J Anaesth 2004; 51: 417–24
- Pinto V, Baldonedo R, Nicolas C, Barez A, Perez A, Aza J. Relationship of transfusion and infectious complications after gastric carcinoma operations. Transfusion 1991; 31: 114–8
- Triulzi DJ, Vanek K, Ryan DH, Blumberg N. A clinical and immunologic study of blood transfusion and postoperative bacterial infection in spinal surgery. Transfusion 1992; 32: 517–24
- Wobbes T, Joosen KH, Kuypers HH, Beerthuizen GI, Theeuwes GM. The effect of packed cells and whole blood transfusions on survival after curative resection for colorectal carcinoma. Dis Colon Rectum 1989; 32: 743–8
- Blumberg N, Heal J, Chuang C, Murphy P, Agarwal M. Further evidence supporting a cause and effect relationship between blood transfusion and earlier cancer recurrence. Ann Surg 1988; 207: 410–5
- Bentzen SM, Balslev I, Pedersen M, Teglbjaerg PS, Hanberg-Sorensen F, Bone J. Blood transfusion and prognosis in Dukes’ B and C colorectal cancer. Eur J Cancer 1990; 26: 457–63
- Fung M, Rao N, Rice J, Ridenour M, Mook W, Triulzi D. Leukoreduction in the setting of open heart surgery: A prospective cohort-controlled study. Transfusion 2004; 44: 30–5
- Hebert P, Ferguson D, Blajchman M, Wells G, Kmetic A, Coyle D, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA 2003; 289: 1941–9
- Vamvakas EC, Carven JH. Allogeneic blood transfusion, hospital charges, and length of hospitalization: A study of 487 consecutive patients undergoing colorectal cancer resection. Arch Pathol Lab Med 1998; 122: 145–51
- Penn I. Depressed immunity and the development of cancer. Cancer Detect Prev 1994; 18: 241–52
- Cerhan JR, Wallace RB, Folsom AR, Potter JD, Munger RG, Prineas RJ. Transfusion history and cancer risk in older women. Ann Intern Med 1993; 119: 8–15
- Skanberg J, Frisk B. Blood transfusion does not influence the development of malignant tumours. Eur J Surg 1999; 165: 528–34