Abstract
Data on side effects after radiotherapy is needed to establish the benefits and drawbacks of new treatments, but side effects are not quantified as easily as survival or local control. Side effects may be quantified using physical measures. Unfortunately, only few endpoints exist where a physical measure is obtainable, and the case of a patient-relevant measure is even rarer. Radiotherapy is often followed by complex symptoms not easily quantifiable by the observer. Quantitative patient reported side effects can be retrieved using validated questionnaires, but this kind of data is often difficult to interpret and the correlation with clinically observable or measurable changes not straightforward.
The exploitation of the possibilities of highly conformal radiotherapy and multimodality treatment depends on a better understanding of the correlation between dose, volume, modifying factors, and side effects. Using pharynx cancer as an example, the purpose of this article is to summarize the possibilities and limitations of different methods for measurement of radiotherapy-induced side effects.
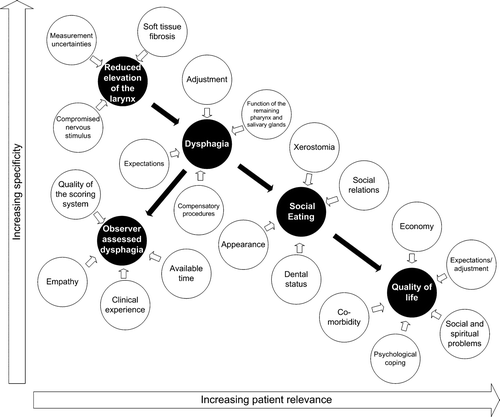
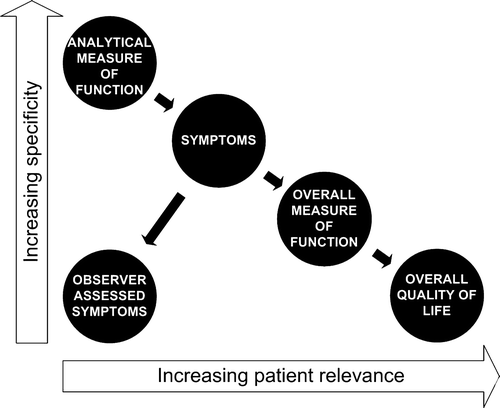
A general agreement exists on how to report local control and survival. A similar agreement does not exist for the reporting of side effects, as these are not easily quantified unambiguously. Radiotherapy may induce changes in organ function and these changes may produce a physiologic sensation interpreted by the patient in a patient-specific context and subsequently reported by the patient as a symptom. Organ-specific symptoms thus have a patient-specific influence on overall measures of health and well-being. Few side effects are physically measurable, and the physical measures that do exist are often of limited relevance to the patient. This trade-off between patient-relevance and specificity has been described by Bentzen Citation[1]. An example is radiotherapy-induced damage of the salivary glands leading to decreased salivary flow. The degree to which this is registered as a dry mouth depends on prior salivary flow, dental status, and mucosal damage. A dry mouth can seriously impair a patient's ability to eat and speak and may have consequential effects for the patient's employment, financial possibilities, social life, and overall quality of life. Sometimes radiation induces damage to major salivary gland without giving rise to any clinically significant effects either because the sub-mucous glands of the oral cavity have been preserved leading to sufficient lubrication of the mucous membranes between meals or because the patient drinks plenty of water with his meals. In these cases, relatively few subjective symptoms and a limited impact on overall quality of life will be registered. The cause-effect relationship is presented in , which also illustrates the patient-relevance and specificity of the different measures of a side effect.
Figure 1. Illustration of the cause effect chain and the trade off between relevance and specificity of different measures of side effects.
Radiobiology helps us understand the consequences of radiotherapy for normal tissue. Side effects can be induced in several ways. A sufficiently high radiation dose will damage all molecules in the irradiated cells and lead to acute organ dysfunction. This is only observed after accidental high-dose exposure as in acute radiation sickness and possibly during radiotherapy in the case of damage to saliva producing cell Citation[2]. Other types of organ dysfunction are produced by damage to the DNA, which is also the basis for the therapeutic effects of irradiation. This kind of effect often only becomes evident when the cells are replicating. The rate at which side effects are observed is thus dependent on the cell turnover of the specific tissues Citation[3]. Late damage is produced by direct parenchymal cell damage or by hypoperfusion stemming from endothelial cell damage and secondarily by replacement of parenchymal cells with fibroblasts. These late changes are observable as atrophy and fibrosis. This chain of events can lead to severe side effects many years after radiotherapy that makes long-term follow up important.
The clinical effects of radiation-induced cell damage also depend on the organisation of the tissue: If the function of all sub-volumes in an organ is a prerequisite for normal organ function, the organ is said to have a serial organisation. The analogy is an electric wire: If a segment is missing, the wire does not conduct electricity. An anatomical example of this is the spinal cord. If, on the other hand, the organ has a reserve capacity the function of the organ depends on the sum of the function of all sub-volumes. This is called a parallel organisation. Examples of this are the parotid glands, the lungs, and the liver. A part of the organ may be damaged increasing the probability of a degree of side effects (normal tissue complication probability, NTCP) but the organ may retain full function or function above a given threshold. These differences in tissue organisation are important for radiotherapy planning. The side effect of an organ organized in series is best predicted by the maximum dose (to a small volume). The mean or median dose, or the volume receiving more than a threshold dose, may predict the risk of side effects of an organ organized in parallel. Mathematical models have been developed to describe the correlation between a heterogeneous dose distribution to an organ and the probability of side effects, depending on the radiosensitivity and volume dependency (organisation) of the organ. Nevertheless, there is not sufficient data to suggest superiority of one model over another Citation[4–8]. It nevertheless seems that these models are needed as inverse dose optimisation based on single parameter-constraints often leads to dose plans giving significant dose to a significant volume just below the constraint value. Dose-volume histogram parameters (DVH) are closely correlated so coincidence often determines which parameter is significant in the single reports Citation[9]. The result is dose distributions with little biological sense, and this approach will probably not result in an optimal reduced chance of avoiding morbidity
The purpose of this article is to summarize the possibilities and limitations of different methods of measuring side effects after radiotherapy. Pharynx cancer is used to as an example to illustrate the challenges.
General methods for measurement of side effects
Radiotherapy can affect organ function in ways that are physically measurable as changes of e.g. organ weight, flow, and biomechanical properties, potentially leading to overall changes in overall well-being and function. The methods for measuring side effects must cover this range of consequences in order to give a comprehensive insight into the side effects of radiotherapy. Scoring manuals for side effects have been developed-the most recent being CTCAE Citation[10]. These manuals contain an abundance of graded endpoints. They are constructed by consensus among researchers and clinicians and are rarely validated against other endpoints. Most scales are based on an observer based scoring of symptom intensity or as registration of initiated treatment of a given side effect. Although grade III-IV morbidity seems to be the standard unit for reporting morbidity, irrespective of endpoint and scoring systems, the different scoring systems have only limited correlation with each other and cannot be used interchangeably Citation[11]. Nevertheless, toxicity should be scored according to generally accepted scoring systems, preferably CTCAE, as these systems are the product of consensus between important scientific organisations, and as uniformity in the reporting of side effects strongly increases the value of data. However, some arguments for not only registering side effects according to CTCAE are discussed in the following.
Objective signs
Semi-quantitative objective assessment scales are available for a variety of endpoints. Several classical radiobiological endpoints belong to this category: Fibrosis, atrophy, and mucositis. Functional endoscopic evaluation of swallowing without (FEES) or with sensory testing (FEESST) can be used to evaluate swallowing Citation[12–14]. Interpretation of auditory evoked potentials Citation[15], speech Citation[16], and cognitive changes Citation[17] are other examples of semi-quantitative endpoints relevant for research in side effects after radiotherapy. The WHO and Karnofsky performance status scales are observer-based estimations of the function and physical capabilities of a patient. Both have been shown to be strong predictors of survival.
Analytical endpoints
Saliva flow is an obvious measure of salivary gland function. Whole mouth and parotid gland flow are measured using either stimulated or resting flow rate. The choice of measure is often based on concerns regarding reproducibility or resources rather than the scientific question posed. Regional assessment of salivary gland function using SPECT or PET and its correlation with radiation dose is an interesting research topic Citation[18], Citation[19]. Since both sub-volume and overall organ function can be measured directly, the dose-volume-effect relationship can theoretically be described without making any model assumptions. Swallowing is traditionally assessed using modified barium swallows (video-fluoroscopy (VF)). It provides the observer with several possibilities of retrieving quantitative measurements of speed and range of motion of the structures involved in the swallowing process as well as a quantitative estimate of residuals, penetration, and aspiration Citation[20].
Observer-scored subjective symptoms
Systems for quantitatively scoring subjective and objective morbidity have been developed and are continuously evolving: Dische Citation[21], WHO Citation[22], NCIC-CTG, EORTC/RTOG SOMA-LENT Citation[23–25], CTCAE Citation[10] and DAHANCA Citation[26] are examples of authors or organisations that have included subjective symptoms in their scoring systems. As mentioned above the development of the systems rests on experience and consensus rather than validated scales. Yet, they are the cornerstone of the majority of available knowledge on subjective side effects after radiotherapy as they are relatively simple, fast, and cheap to use. The scoring systems are sensitive enough to detect differences in toxicity dependent on volume Citation[27], acceleration Citation[28], fractionation Citation[29], and concomitant chemotherapyCitation[11].
Patient-assessed symptoms and quality of life
Several patient-administered, well-validated questionnaires for cancer patients exist. Fortunately, head and neck cancer is almost as popular among quality of life researchers as it is among radiobiologists: Several head and neck cancer as well as symptom-specific questionnaires are available. Examples are presented in . As for observer-assessed morbidity, the key issue is to pick a well-validated measurement tool that is well known to the scientific community, and as for observer-assessed morbidity, it is also the case that two methods for measurement should not be expected to produce comparable scores just because the name of their scales might be identical Citation[30].
Table I. Important available tools for retrieving patient-reported morbidity data.
Specific side effects after radiotherapy for pharynx cancer
Reduction in quality of life and three different side effects will be mentioned to illustrate the methodological difficulties of measuring side effects: Swallowing problems are mentioned as an example where no agreement exists on the relevant endpoint, the organ at risk, or the preferred objective analytical method. Dry mouth is mentioned as an example of a side effect with a relatively simple analytical endpoint and a well-defined organ at risk. Finally, dental problems may be objectively well defined, but no analytical endpoints exist and its causal relation with radiotherapy is insufficiently described.
Dysphagia
Dysphagia is the sensation in a patient of having problems with eating and swallowing. It is not reported to be as intense or frequent as xerostomia, but it might be of greater importance for health Citation[31]. Dysphagia prolongs or prohibits the intake of normal meals. It thereby impacts on the social life of the patients. Since the patients tend to eat less and to limit the variation of food, they are at risk of becoming under- and malnourished. Some patients become dependent on a feeding tube, some of them even for life. Part of the function of the swallowing reflex is to prevent aspiration, i.e. the entry of liquid and food into the airways. Aspiration puts the patient at risk for repetitive pneumonias and perhaps even death Citation[32]. However, little is known about the clinical importance of aspiration. It is a frequent finding in long-time survivors but it often goes unnoticed Citation[33–35]. During combined modality treatment where chemotherapy intensifies the effects of radiotherapy, dysphagia is often described as the dose-limiting side effect Citation[32], Citation[36].
Swallowing is a complex process that involves many structures. The food is chewed in the mouth where it is also mixed with saliva to form a bolus. The bolus is swallowed consciously when the tongue is pressed up- and backwards to initiate a row of reflexes. The soft palate is moved cranially closing the nasopharynx. Then the base of the tongue and posterior pharyngeal wall (the upper pharyngeal constrictor) is moved together to propel the bolus downwards. This pushes the epiglottis down- and backwards. Simultaneously, the larynx moves cranially and the vocal cords close to protect the airways. The upper oesophageal sphincter is relaxed, and the lower part of the pharyngeal constrictor presses the bolus into the oesophagus. The bolus then passes through the oesophagus by a peristaltic movement of involuntary muscles. This is a carefully orchestrated process depending on connective tissue, muscles, motor, and sensory nerves. All of these structures can be damaged by radiotherapy, and many objective changes have been identified after radiotherapy: Reduced range of motion and decreased speed of movement of the tongue, the base of the tongue, the posterior pharyngeal wall, larynx, the vocal cords, and the upper oesophageal sphincter. Also, compromise of composite measures of swallowing have been described: Velopharyngeal incompetence, delayed swallowing reflex, reduced OPSE (oropharyngeal swallowing efficiency), pyriform sinus and valecular residuals, premature leakage and reduced sensitivity Citation[37–41].
The standard swallowing examination is the modified barium swallow (video-fluoroscopy (VF)). It provides the observer with the possibility of retrieving quantitative measurements of speed and range of motion of the structures involved in the swallowing process as well as a quantitative estimate of residuals, penetration and aspiration Citation[20].
Functional endoscopic evaluation of swallowing (FEES) without or with sensory testing (FEESST) can also be used Citation[12–14]. It does not produce direct information on the oropharyngeal phase of swallowing but this can be assessed indirectly. Furthermore, the method gives information on the sensitivity of the throat and aspiration of saliva. It is cheaper than VF and does not expose the patient to ionising radiation Citation[14], Citation[20]. The output of the examination is unfortunately only semi-quantitative measures.
Only few researchers have tried to correlate tumour dose with dysphagia. Smith et al. Citation[42] report from a non-randomized study in 27 patients treated with 60 or 74.4 Gy tumour dose concomitant with chemotherapy. They found more penetration/ aspiration and more frequent long-term tube dependency with 74.4 Gy than with 60 Gy. A study by Wu Citation[39] has been presented including FEES data that failed to show a correlation between tumour dose and dysphagia.
The organ at risk for the development of dysphagia is a matter of debate. No single organ or specific dysfunction has been shown to determine the overall swallowing process and the ability for protection of airways. Eisbruch Citation[43] has presented a study of 26 patients receiving chemo-radiation. Videoflouroscopy (VF) abnormalities were seen in all phases of the swallows. Based on significant oedema on CT scans the pharyngeal constrictor, supraglottic larynx, and glottic larynx were identified as the dysphagia-/aspiration-related structures. A continuation of this study has been conducted, and the results of an IMRT protocol aimed at sparing the dysphagia-related structures have been presented by Feng ASTRO 2006 Citation[44]. Even after deliberately reducing radiation dose to the dysphagia-related structures, dose was still predictive of changes on the VF. The relation between dose to organs at risk and swallowing function has also been described in an abstract from ASTRO 2005: Simmons et al. Citation[45] presented data on 27 patients. Patient-reported diet, swallowing, and speech data showed a significant correlation with doses to the aryepiglottic fold, false vocal cords, and lateral pharyngeal walls at the level of the false cords. Levendag et al. presented data on subjective swallowing in 77 head and neck cancer patients at ESTRO 2006 Citation[46]. Mean doses of potential organs at risk were used in the analysis, and doses to the upper and median pharyngeal constrictors significantly correlated with the side effects reported in quality of life questionnaires. Jensen et al. have investigated the dependency on dose to critical structures of the upper aerodigestive tract of answers to the swallowing-related scales in the EORTC H&N35 questionnaire as well as of the result of a FEES. Dose-volume parameters of especially the supraglottic region were predictive of swallowing dysfunction Citation[33].
Even though relevant endpoints have been chosen for these studies, there is no agreement as to which organ should be spared. This can be explained by the diversity in examination methods and endpoints. Further studies are needed that take into account the effect of primary tumour site. Ideally, the function of sub-structures (e.g. the ary-region) should be evaluated and compared with doses to these volumes, and models should be constructed to predict overall changes, e.g. aspiration, weight loss, normality of diet or dysphagia based on dose-volume parameters of the sub-structures and their function.
It is believed that dysphagia can be partially avoided or treated with exercises Citation[47], Citation[48]. Little evidence supports this belief Citation[49] however. The exercise strategy has the advantage as compared with an organ-sparing strategy that it does not influence radiotherapy planning and specifically does not introduce a risk of underdosage of the tumour. At Aarhus University Hospital a study has been initiated of prophylactic swallowing exercises (ClinicalTrials.gov Identifier: NCT00332865).
The cause-effect relationship between different measures of swallowing dysfunction and selected contributing factors is illustrated in . The figure does not provide a full explanation of the swallowing process but is intended to be illustrative of the fact that specificity of the endpoint must decrease as more factors may explain an endpoint Similar figures can be constructed for all endpoints.
Dry mouth
The subjective feeling of dryness of the mouth, xerostomia, has been pointed out by the patients as the most frequent and bothering side effect after radiotherapy for head and neck cancer Citation[50]. Xerostomia can lead to problems with speaking for longer periods without sipping water. Saliva facilitates chewing and bolus formation of the food, and flavours must be dissolved in fluid before they can be tasted. Patients with xerostomia often have problems sleeping because of dry mucous membranes, especially if they have to breath through the mouth, e.g. during a cold. The same is often a hindrance to strenuous physical activity. Saliva physically flushes the teeth and possesses pH-buffering as well as anti-microbial activities. Saliva is therefore important for dental status Citation[51].
Parotid gland sparing has been an important argument for introducing IMRT in many departments of radiotherapy. Important clinical results have been published demonstrating high local control rates and preserved parotid function at the same time Citation[52], Citation[53]. Several authors have contributed with data to establish a dose-volume dependency of the parotid. A mean dose to these structures below 26–30 Gy seems to result in a low probability of xerostomia Citation[18], Citation[54–57]. The parotid gland is a good example of a well-defined structure (the gland) with a well defined endpoint (saliva production), making it an ideal test case for volume sparing. As suggested in the introduction, a mean dose below a fixed threshold will probably not be the perfect consbstraint for xerostomia. Mean doses as a single -constraint parameter are counter-intuitive because a very high dose to a small area will affect the parameter more than is biologically reasonable-cells can only be killed once. At the same time, glandular structures treated to low doses are capable of compensatory hypertrophy. Treatment of xerostomia is often very difficult, and most patients must substitute saliva with either water or artificial saliva. If some salivary function is preserved after radical radiotherapy, salivary secretion can be stimulated with drugs, acidic candy, or chewing gum Citation[58]. Acupuncture has been tested, but with no proven benefit Citation[59]. Furthermore, xerostomia can partially be prevented using amifostine, a radioprotectant with a relative specificity to normal tissues, but with a high degree of acute side effects Citation[60].
Several relevant endpoints of salivary gland functions exist. Saliva flow can be measured as whole mouth flow, either by having the patient spitting in a pre-weighted cup, by placing a pre-weighted cotton cloth in the mouth, by suction, and by draining Citation[61]. This will collect saliva from the macroscopic salivary glands and from the sub-mucous glands. Alternatively, saliva can be collected more gland-specific by placing collecting tubes over the orifices of the parotids Citation[62] or sublingual/ submandibular glands Citation[63]. Gland-specific flow measurements are essential for establishing dose-volume-effect relationships, as other measures will depend on several variables. The salivary glands are stimulated by a nervous signal. This can be stimulated by a parasympathomimetic agent such as pilocarpine, by having the patients chewing on taste-less paraffin, or by applying a sour substance on the tongue Citation[64]. Irrespective of measurement methods, the inter- and intra-subject variations are high, especially for un-stimulated flow Citation[61], Citation[65]. Comparing different methods of stimulation, Ericson Citation[66] found only a moderate correlation. An abstract by Duran Citation[67] reports that by stimulating saliva flow with 2% citric acid, applied to the tongue every 30 seconds, the saliva flow was maximally stimulated after 2 minutes, and this could be maintained. This is in contrast to the varied peak effect of pharmacological substances. Whole-mouth saliva flow is probably more relevant to the patient than gland-specific flow. Unstimulated whole-mouth saliva flow is used as the objective endpoint for xerostomia in CTCAE 3.0. Unstimulated flow probably determines the sensation of dry mouth during sleep and speech as the unstimulated mucous saliva lubricates the mucous membranes between meals. The parotid glands produce more serous saliva, when stimulated, which enables chewing and initiates digestion. This could indicate that stimulated parotid flow is less important for the sensation of xerostomia, since the patients will be able to compensate for the lack of parotids function by drinking during meals. The available data only partly supports this simplistic correlation between salivary gland function and xerostomia. Both whole mouth and parotid saliva flow have been correlated with xerostomia Citation[55], Citation[68–71]. Radiotherapy dose to the parotid and submandibular gland have been correlated with flow as well as xerostomia Citation[57], Citation[69–71]. Of special interest is the study by Eisbruch Citation[71]. He reports that dose to the oral cavity was the best predictor of xerostomia indicating an important role of the lubricating saliva from the submucous glands. However, this could not be confirmed in a subsequent study by Jellema Citation[55].
Dental problems
It is well known that dental problems increase after radiotherapy for head and neck cancer. It is often mentioned, but poorly examined. Head and neck cancer is most often seen in patients above 60 years of age, in smokers, and in patients with a relatively poor socioeconomic status. The same parameters define those at greatest risk for dental problems, even before treatment Citation[31]. Dental problems after radiotherapy are related to salivary gland dysfunction Citation[51]. Few findings support a direct adverse effect of radiotherapy on the dental tissues Citation[72], Citation[73], but periodontal attachment loss has been shown to be more pronounced in the treated side compared with the untreated side in case of unilateral treatment fields Citation[73]. A direct measurement method for dental status is not available. Objective assessment is, however, performed in the routine pre-therapeutic evaluation, as dental status is important for the risk of developing osteoradionecrosis. The overall changes in dental status can be scored according to CTCAE 3.0.
Reduced quality of life
The WHO has defined quality of life (and health) as “a state of complete physical, mental and social well-being, and not merely the absence of disease”. Two lessons can be learned from this very general statement: 1) A strict definition is difficult and maybe even impossible to make. Quality of life is a concept open to individual interpretation in a context that varies with experience, age, gender, and culture. 2) Quality of life is multidimensional. Apart from physical, mental, and social well-being, dimensions such as existential and spiritual well-being could be added Citation[74].
Overall, health related QoL in pharynx cancer patients is, to a large extent, determined by the above-mentioned and other symptoms and their consequences: The treatment may have an effect on smell, taste, appearance, speech, sexuality, mobility of head, neck and arms, breathing, pain, mood, and social interaction. Locally advanced uncontrolled disease gives rise to further symptoms but the description of these lies beyond the scope of the present paper.
Quality of life can be examined with in-depth interviews of a limited number of patients, and a qualitative description of a problem can be given as is for instance the case for acute dysphagia as described by Larsson Citation[75]. To produce a feasible quantitative description, the aim must be simplified. A common way of achieving this is questionnaires. Important themes for a questionnaire must be identified by careful study of the literature and interviews with patients and professionals from multiple disciplines. The endpoints that the researcher wants to elucidate (constructs) must be phrased as questions (items). The most important questions are selected by presenting the questionnaire draft to large patient groups. Items that are too identical to others or touch on a problem too rarely encountered are then deleted. Items can be grouped together to form a scale in order to increase the validity of the answer by asking the questions in different ways, asking about many symptoms of the same construct, different degrees of the symptoms, or several dimensions of the construct. Scales can be constructed based on the statistical behaviour of the items (factor analysis) or based on the constructs (clinical “common sense”). The questionnaire is tested again. The purpose of the second test is to confirm the scale validity and to examine if the questionnaire is sensitive to differences between patients with e.g. different tumour load and sensitive to changes over time with e.g. tumour progression. If the questionnaire is to be used in a population that differs with respect to age, types of encountered problems, cultural background and, not least language, the questionnaire must be retested and perhaps adapted. This is obviously a lengthy and costly process. The result should, nevertheless, be a valid and reliable questionnaire that can measure what was hitherto immeasurable or measurable with poorer sensitivity or specificity.
One of the endpoints of the questionnaire is often overall (health-related) quality of life. This quantity is defined in the EORTC questionnaire by two questions asking the patients to rate overall health and overall quality of life. No definitions are given to the patients, and the score represents the normalized mean of these two questions Citation[76]. Other systems use mean score of all (un-related) items of the questionnaire as a measure of overall quality of life Citation[77] or head and neck specific quality of life Citation[78]. The interpretation of these scores does not seem to be easier than the “undefined” score of EORTC. The more composite the endpoint is, the harder does it seem to define it, and the more is it dependent on other factors than the ones studied Citation[79] ( and ). For instance, differences over time or between cancer survivors and the overall population might not be significant for several important overall endpoints Citation[80]. The absence of expected differences has to do with coping, or response shift, i.e. changes in values and expectations as well as re-conceptualization Citation[81]. Nevertheless, a side effect that the patient has gotten used to is still a side effect.
Comparison of endpoints
Endpoints can be compared with respect to response rates, tolerability, and resources. This is not provided in the present paper. Tools can also be compared if a gold standard exists. It is hard to argue that the gold standard for subjective endpoints must be the patient-assessed side effect. If a difference between patient groups is hypothesized, the sensitivity of different methods of morbidity assessment to detect this difference can be examined.
Stephens et al. Citation[82] compared physician ratings with patient-assessed symptoms using the Rotterdam Symptom Checklist in two randomized controlled trials. Physicians tended to underestimate symptoms, but the degree of underestimation varied greatly between symptoms and between studies (treatment modality), but did not vary with the number of patients seen per centre, indicating that training was not the reason for underestimation. An interesting finding was that concordance decreased as patient-reported symptom intensity increased. The comparison of toxicity between treatment arms was, nevertheless, consistent irrespective of data collection method. Basch Citation[83] compared clinician and patient rating using an adapted form of the CTCAE in an out-patient population. Again, there was a tendency for physicians to underestimate symptoms, especially more subjective, unobservable endpoints. No patient or observer variables were associated with the disagreement.
Using different scoring systems has yielded similar results: Answers to the EORTC H&N35 have been compared with equivalent endpoints of the DAHANCA scoring system in 116 recurrence-free head and neck cancer survivors attending follow up Citation[84]. The clinicians severely underestimated the complaints, especially regarding xerostomia. The questionnaire endpoints, but not the observer-based scores, were sensitive to the detrimental effects of smoking Citation[85]. Of note is that patient-assessed physical function using questions resembling the WHO performance status (PS) was more closely associated with observer-assessed (and patient-assessed) toxicity than PS. Meirovitz Citation[86] compared the RTOG/EORTC xerostomia scores with patient-assessed xerostomia in 38 patients using a xerostomia questionnaire and found no correlation but a severe underestimation of xerostomia by the observers. Bjordal has also found an underestimation of frequency and intensity of symptoms in head and neck cancer patients in a cross sectional study Citation[87]. Homsi found a 10-fold increase in the number of reported symptoms in a population of palliative patients using a checklist compared to open-ended questions Citation[88]. Movsas analyzed the results of a randomized study with amifostine in lung cancer patients treated with chemo-radiotherapy Citation[89]. The observer-based scoring of side effects was sensitive enough to detect the acute toxicity of amifostine, but not reduced dysphagia. On the other hand, patient-assessed swallowing diaries showed a significant effect of amifostine at week 8 and the pain item of the EORTC C30 form were improved at week 6. Whether this can be translated into a clinical benefit is another question, but it proves that sensitivity to detect treatment effects can be increased if the proper tool is chosen.
Studies on other endpoints have been performed using observer- and patient-based systems. Objective assessment of cognitive function and sedation, using Mini Mental State Examination and Alertness/Sedation scale, were not correlated with self-reported cognitive function symptoms of the EORTC questionnaire in 29 palliative patients Citation[90]. Patient-reported swallowing problems have been compared with FEES findings, dental problems with a dental examination, and xerostomia with saliva flow in 35 pharynx cancer survivors Citation[31]. A significant correlation for all endpoints was found, but patient-reported toxicity only predicted observable morbidity with a sensitivity of 0.60–0.83, a specificity of 0.43–0.81, a positive predictive value of 0.28–0.81 and a negative predictive value of 0.46–0.94. Thus, also these results were very variable. In a chemotherapy trial of prostate cancer, Fromme Citation[91] compared clinician-reported treatment-related adverse effects on CTC-NCI with increasing (>10 points) symptoms on the EORTC C30 questionnaire and found a sensitivity of 24–83% and a specificity of 9–92%, again using QoL as the gold standard.
Patient-reported QoL has been analyzed as a predictor for local control and survival in head and neck cancer. Endpoints as fatigue Citation[92] and cognitive function Citation[93] have been statistically significant, and superior to physician-assessed overall physical function (Karnofsky performance scale and WHO performance status (PS).
Another measurement of sensitivity is to assess if the measurement tool can be used for establishing dose-volume effect correlations in radiotherapy. As mentioned above observer-scored endpoints have been correlated with all the important parameters of radiobiology. The dose-volume effect correlation for swallowing problems has been examined, and the authors Citation[33] were able to correlate dose and volume parameters of several potential organs at risk to both patient-assessed swallowing problems and objective changes assessed by endoscopy (FEES), but not to observer-assessed dysphagia.
Sensitivity to the detection of a beneficial or harmful effect is one measure of the quality of an endpoint, but do quality of life studies impact on the interpretation of study results? This question has not been addressed in head and neck cancer, but reports have evaluated the results of studies in breast cancer Citation[94], prostate cancer Citation[95], and surgical oncology Citation[96]. The general conclusions are that after well-performed studies, quality of life data has an impact on decision making after studies without proven survival benefit of one arm. Furthermore, quality of life data often adds to the knowledge of side effects and improves the quality of information that can be offered to future patients.
Conclusion and recommendations
To evaluate the therapeutic gain of a certain treatment, knowledge of both sides of the coin must be collected. Local control and survival as well as side effects must be quantified in clinical studies. Not only must the relevant endpoints of morbidity be quantified and registered, but the data must also be analysed and presented in a relevant manner. Actuarial analysis has been suggested to be the method of choice for analysing and reporting late effects Citation[97], Citation[98]. It is certainly to be preferred to simple frequencies of late effects, but some endpoints, such as acute toxicity and late xerostomia are at least partially reversible, and therefore not suited for actuarial analysis Citation[99–102]. Items that must be discussed before toxicity data is collected are mentioned in .
Table II. Questions to ask before selecting methods for measuring side effects.
The correlation between physiologic changes, symptoms, and a detrimental effect on quality of life is substantiated by the literature for many endpoints, but a measurement of one of the terms of the cause-effect chain cannot be replaced by quantifying another term without loosing specificity or sensitivity. If a treatment or prevention is aimed at inducing a certain physiologic effect in the patient that can be measured, this measurement should be carried out, as it will establish the proof of principle with the greatest specificity and sensitivity. For quantifying subjective symptoms, validated quality of life questionnaires exist that includes questions on most side effects. Evidence is available that quality of life data has impact on the interpretation of trial results and other data is available showing that the data can be retrieved without insurmountable problems e.g. using touch screens in the patient waiting areas Citation[103], Citation[104]. Therefore, physician-based scoring of subjective symptoms can only be justified by arguments of resources since it lacks specificity and sensitivity.
In order to gain the full benefit of the possibilities of more conformal radiotherapy, more specific morbidity data must be collected with the best available methods to gain information on dose and volume dependency. At the same time, radiotherapy is more often combined in multimodality treatments. Each modality has its unique morbidity profile and new side effects will arise as a consequence of the combination of modalities. This poses new demands for methods that assess the overall strain on the patient and at the same time is adaptable and sensitive enough to allow registration of unexpected specific toxicities. All information must be collected prospectively and interpreted in the light of duration, progression, or reversibility. These challenges raise a number of important scientific and logistical questions that await an answer.
References
- Bentzen SM, Dorr W, Anscher MS, Denham JW, Hauer-Jensen M, Marks LB, et al. Normal tissue effects: Reporting and analysis. Semin Radiat Oncol 2003; 13: 189–202
- Hakim SG, Jacobsen HC, Hermes D, Kosmehl H, Lauer I, Nadrowitz R, et al. Early immunohistochemical and functional markers indicating radiation damage of the parotid gland. Clin Oral Investig 2004; 8: 30–5
- Steel GG, Begg AC, Stewart FA, van der Kogel AJ, Joiner MC, McMillan TJ, et al. Basic Clinical Radiobiology3rd ed. Arnold, London 2002; 26
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109–22
- Lyman JT. Normal tissue complication probabilities: Variable dose per fraction. Int J Radiat Oncol Biol Phys 1992; 22: 247–50
- Kwa SL, Theuws JC, Wagenaar A, Damen EM, Boersma LJ, Baas P, et al. Evaluation of two dose-volume histogram reduction models for the prediction of radiation pneumonitis. Radiother Oncol 1998; 48: 61–9
- Niemierko A, Goitein M. Calculation of normal tissue complication probability and dose-volume histogram reduction schemes for tissues with a critical element architecture. Radiother Oncol 1991; 20: 166–76
- Kutcher GJ. Quantitative plan evaluation: TCP/NTCP models. Front Radiat Ther Oncol 1996; 29: 67–80
- Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 2006.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–81
- Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: Comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int J Radiat Oncol Biol Phys 2003; 55: 93–8
- Aviv JE, Kaplan ST, Thomson JE, Spitzer J, Diamond B, Close LG. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): An analysis of 500 consecutive evaluations. Dysphagia 2000; 15: 39–44
- Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. Laryngoscope 2000; 110: 563–74
- Wu CH, Hsiao TY, Chen JC, Chang YC, Lee SY. Evaluation of swallowing safety with fiberoptic endoscope: Comparison with videofluoroscopic technique. Laryngoscope 1997; 107: 396–401
- Grau C, Moller K, Overgaard M, Overgaard J, Elbrond O. Auditory brain stem responses in patients after radiation therapy for nasopharyngeal carcinoma. Cancer 1992; 70: 2396–401
- Pauloski BR, Rademaker AW, Logemann JA, Colangelo LA. Speech and swallowing in irradiated and nonirradiated postsurgical oral cancer patients. Otolaryngol Head Neck Surg 1998; 118: 616–24
- Cheung M, Chan AS, Law SC, Chan JH, Tse VK. Cognitive function of patients with nasopharyngeal carcinoma with and without temporal lobe radionecrosis. Arch Neurol 2000; 57: 1347–52
- Buus S, Grau C, Munk OL, Rodell A, Jensen K, Mouridsen K, Keiding S. Individual radiation response of parotid glands investigated by dynamic 11C-methionine PET. Radiother Oncol 2006; 78: 262–9
- van Acker F, Flamen P, Lambin P, Maes A, Kutcher GJ, Weltens C, et al. The utility of SPECT in determining the relationship between radiation dose and salivary gland dysfunction after radiotherapy. Nucl Med Commun 2001; 22: 225–31
- Langmore SE. Evaluation of oropharyngeal dysphagia: Which diagnostic tool is superior?. Curr Opin Otolaryngol Head Neck Surg 2003; 11: 485–9
- Dische S, Warburton MF, Jones D, Lartigau E. The recording of morbidity related to radiotherapy. Radiother Oncol 1989; 16: 103–8
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6
- Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, et al. EORTC Late Effects Working Group. Late effects toxicity scoring: the SOMA scale. Radiother Oncol 1995; 35: 11–5
- Rubin P, Constine LS, III, Fajardo LF, Phillips TL, Wasserman TH. EORTC Late Effects Working Group. Overview of late effects normal tissues (LENT) scoring system. Radiother Oncol 1995; 35: 9–10
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003; 20;362: 933–40
- Jensen K, Overgaard M, Grau C. Morbidity after Ipsilateral radiotherapy for oropharyngeal cancer. Radiother Oncol 2007. In press.
- Skladowski K, Maciejewski B, Golen M, Tarnawski R, Slosarek K, Suwinski R, et al. Continuous accelerated 7-days-a-week radiotherapy for head-and-neck cancer: Long-term results of phase III clinical trial. Int J Radiat Oncol Biol Phys 2006; 66: 706–13
- Dische S, Saunders M, Barrett A, Harvey A, Gibson D, Parmar M. A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol 1997; 44: 123–36
- Kemmler G, Holzner B, Kopp M, Dunser M, Margreiter R, Greil R, et al. Comparison of two quality-of-life instruments for cancer patients: The functional assessment of cancer therapy-general and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30. J Clin Oncol 1999; 17: 2932–40
- Jensen K, Lambertsen K, Torkov P, Dahl M, Jensen AB, Grau C. Patient assessed symptoms are poor predictors of objective findings. Results from a cross sectional study in patients treated with radiotherapy for pharynx cancer. Acta Oncol In press.
- Eisbruch A. Dysphagia and aspiration following chemo-irradiation of head and neck cancer: Major obstacles to intensification of therapy. Ann Oncol 2004; 15: 363–4
- Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer. Frequency, intensity and correlation with dose and volume parameters. Radiather Oncol 2007 In press.
- Nguyen NP, Frank C, Moltz CC, Vos P, Smith HJ, Bhamidipati PV, et al. Aspiration rate following chemoradiation for head and neck cancer: An underreported occurrence. Radiother Oncol 2006.
- Campbell BH, Spinelli K, Marbella AM, Myers KB, Kuhn JC, Layde PM. Aspiration, weight loss, and quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg 2004; 130: 1100–3
- Robbins KT. Barriers to winning the battle with head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002; 53: 4–5
- Lazarus CL. Effects of radiation therapy and voluntary manoeuvres on swallow functioning in head and neck cancer patients. Clin Commun Disord 1993; 3: 11–20
- Shaker R, Easterling C, Kern M, Nitschke T, Massey B, Daniels S, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology 2002; 122: 1314–21
- Wu CH, Hsiao TY, Ko JY, Hsu MM. Dysphagia after radiotherapy: Endoscopic examination of swallowing in patients with nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol 2000; 109: 320–5
- Parise JO, Miguel RE, Gomes DL, Menon AD, Hashiba K. Laryngeal sensitivity evaluation and dysphagia: Hospital Sirio-Libanes experience. Sao Paulo Med J 2004; 122: 200–3
- Hughes PJ, Scott PM, Kew J, Cheung DM, Leung SF, Ahuja AT, et al. Dysphagia in treated nasopharyngeal cancer. Head Neck 2000; 22: 393–7
- Smith RV, Goldman SY, Beitler JJ, Wadler SS. Decreased short- and long-term swallowing problems with altered radiotherapy dosing used in an organ-sparing protocol for advanced pharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2004; 130: 831–6
- Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT?. Int J Radiat Oncol Biol Phys 2004; 60: 1425–39
- Feng FY, Kim HM, Lyden T, Haxer MJ, Feng M, Worden FP, Chepeha DB, Eisbruch A. Intensity-modulated radiatherapy of head and neck cancer aiming to reduce dysphagia: Early relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007 Jun 6; [Epub ahead of print].
- Simmons JR, Dornfeld K, Karnell M, Funk G, Yao M, Wacha B, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long term diet and speech related quality of life. Int J Radiat Oncol Biol Phys 2005; 63: S132
- Levendag P, Teguh D, van Rooil P, Voet P, van der Est H, Heijmen B, et al. Dysphagia related quality of life patients with cancer in the oropharynx is significantly affected by radiation therapy dose received by the superior- and middle constrictor mucle: A dose effect relationship. Radiother Oncol 2006; 81(Suppl 1)S337–S338
- Mittal BB, Pauloski BR, Haraf DJ, Pelzer HJ, Argiris A, Vokes EE, et al. Swallowing dysfunction–preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: A critical review. Int J Radiat Oncol Biol Phys 2003; 57: 1219–30
- Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 2006; 24: 2636–43
- Carnaby-Mann G. Preventive exercise for dysphagia following head and neck cancer. Dysphagia Symposium, EGDG Meeting 2006, Sweden, 2006. p 46–7. (Abstract).
- Jensen AB, Hansen O, Jorgensen K, Bastholt L. Influence of late side-effects upon daily life after radiotherapy for laryngeal and pharyngeal cancer. Acta Oncol 1994; 33: 487–91
- Bardow A, ten Cate JM, Nauntofte B, Nyvad B. Effect of unstimulated saliva flow rate on experimental root caries. Caries Res 2003; 37: 232–6
- de Arruda FF, Puri DR, Zhung J, Narayana A, Wolden S, Hunt M, et al Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: The memorial sloan-kettering cancer center experience. Int J Radiat Oncol Biol Phys 2005.
- Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002; 53: 12–22
- Blanco AI, Chao KS, El N, I, Franklin GE, Zakarian K, Vicic M, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:1055–69.
- Jellema AP, Doornaert P, Slotman BJ, Leemans CR, Langendijk JA. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy?. Radiother Oncol 2005; 77: 164–71
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 45: 577–87
- Saarilahti K, Kouri M, Collan J, Kangasmaki A, Atula T, Joensuu H, Tenhunen M. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol 2006; 78: 270–5
- Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced Xerostomia in patients with head and neck cancer: Pathogenesis, impact on quality of life, and management. Head Neck 2004; 26: 796–807
- Wong RK, Jones GW, Sagar SM, Babjak AF, Whelan T. A Phase I-II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 472–80
- Wasserman TH, Brizel DM, Henke M, Monnier A, Eschwege F, Sauer R, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys 2005; 63: 985–90
- Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res 1982; 61: 1158–62
- Jones RE, Takeuchi T, Eisbruch A, D'Hondt E, Hazuka M, Ship JA. Ipsilateral parotid sparing study in head and neck cancer patients who receive radiation therapy: Results after 1 year. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81: 642–8
- Tylenda CA, Ship JA, Fox PC, Baum BJ. Evaluation of submandibular salivary flow rate in different age groups. J Dent Res 1988;67:1225–8 (Abstract).
- Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993; 694: 72–7
- Burlage FR, Pijpe J, Coppes RP, Hemels ME, Meertens H, Canrinus A, et al. Variability of flow rate when collecting stimulated human parotid saliva. Eur J Oral Sci 2005; 113: 386–90
- Ericson S. An investigation of human parotid saliva secretion rate in response to different types of stimulation. Arch Oral Biol 1969; 14: 591–6
- Duran V, Dominguez P, Morales I, Lopez RO. Kinetic assessment of salivary secretory response to citric acid. Differences with pilocarpine. Rev Med Chil 1998;126:1330–7 (Abstract)
- Amosson CM, Teh BS, Van TJ, Uy N, Huang E, Mai WY, et al. Dosimetric predictors of xerostomia for head-and-neck cancer patients treated with the smart (simultaneous modulated accelerated radiation therapy) boost technique. Int J Radiat Oncol Biol Phys 2003; 56: 136–44
- Parliament MB, Scrimger RA, Anderson SG, Kurien EC, Thompson HK, Field GC, et al. Preservation of oral health-related quality of life and salivary flow rates after inverse-planned intensity- modulated radiotherapy (IMRT) for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004; 58: 663–73
- Roesink JM, Schipper M, Busschers W, Raaijmakers CP, Terhaard CH. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: Implications for future trials. Int J Radiat Oncol Biol Phys 2005; 63: 1006–9
- Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 50: 695–704
- Springer IN, Niehoff P, Warnke PH, Bocek G, Kovacs G, Suhr M, et al. Radiation caries–radiogenic destruction of dental collagen. Oral Oncol 2005; 41: 723–8
- Epstein JB, Lunn R, Le N, Stevenson-Moore P. Periodontal attachment loss in patients after head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 86: 673–7
- Efficace F, Marrone R. Spiritual issues and quality of life assessment in cancer care. Death Stud 2002; 26: 743–56
- Larsson M, Hedelin B, Athlin E. Lived experiences of eating problems for patients with head and neck cancer during radiotherapy. J Clin Nurs 2003; 12: 562–70
- Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, et al. Quality of life in head and neck cancer patients: Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol 1999; 17: 1008–19
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol 1993; 11: 570–9
- Browman GP, Levine MN, Hodson DI, Sathya J, Russell R, Skingley P, et al. The Head and Neck Radiotherapy Questionnaire: A morbidity/quality-of-life instrument for clinical trials of radiation therapy in locally advanced head and neck cancer. J Clin Oncol 1993; 11: 863–72
- King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 1996; 5: 555–67
- Pourel N, Peiffert D, Lartigau E, Desandes E, Luporsi E, Conroy T. Quality of life in long-term survivors of oropharynx carcinoma. Int J Radiat Oncol Biol Phys 2002; 54: 742–51
- Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med 1999; 48: 1531–48
- Stephens RJ, Hopwood P, Girling DJ, Machin D. Randomized trials with quality of life endpoints: Are doctors’ ratings of patients’ physical symptoms interchangeable with patients’ self-ratings?. Qual Life Res 1997; 6: 225–36
- Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. Lancet Oncol 2006; 7: 903–9
- Jensen K, Jensen AB, Grau C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires. Radiother Oncol 2006; 78: 298–305
- Jensen K, Jensen AB, Grau C. Smoking has a negative impact upon health related quality of life after treatment for head and neck cancer. Oral Oncol. 2007 Feb;43(2):187–92. Epub 2006 Jul 24.
- Meirovitz A, Murdoch-Kinch CA, Schipper M, Pan C, Eisbruch A. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006; 66: 445–53
- Bjordal K, Freng A, Thorvik J, Kaasa S. Patient self-reported and clinician-rated quality of life in head and neck cancer patients: A cross-sectional study. Eur J Cancer B Oral Oncol 1995; 31B: 235–41
- Homsi J, Walsh D, Rivera N, Rybicki LA, Nelson KA, Legrand SB, et al. Symptom evaluation in palliative medicine: Patient report vs systematic assessment. Support Care Cancer 2006; 14: 444–53
- Movsas B, Scott C, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation therapy oncology group trial 98–01. J Clin Oncol 2005; 23: 2145–54
- Klepstad P, Hilton P, Moen J, Fougner B, Borchgrevink PC, Kaasa S. Self-reports are not related to objective assessments of cognitive function and sedation in patients with cancer pain admitted to a palliative care unit. Palliat Med 2002; 16: 513–9
- Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 2004; 22: 3485–90
- Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 2004; 100: 425–32
- de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer 2001; 37: 332–9
- Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA. Health-related quality-of-life measurement in randomized clinical trials in breast cancer–taking stock. J Natl Cancer Inst 2003; 95: 263–81
- Efficace F, Bottomley A, Osoba D, Gotay C, Flechtner H, D'haese S, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: A checklist for evaluating HRQOL outcomes in cancer clinical trials–does HRQOL evaluation in prostate cancer research inform clinical decision making?. J Clin Oncol 2003; 21: 3502–11
- Blazeby JM, Avery K, Sprangers M, Pikhart H, Fayers P, Donovan J. Health-related quality of life measurement in randomized clinical trials in surgical oncology. J Clin Oncol 2006; 24: 3178–86
- Bentzen SM, Vaeth M, Pedersen DE, Overgaard J. Why actuarial estimates should be used in reporting late normal-tissue effects of cancer treatment … now!. Int J Radiat Oncol Biol Phys 1995; 32: 1531–4
- Jung H, Beck-Bornholdt HP, Svoboda V, Alberti W, Herrmann T. Quantification of late complications after radiation therapy. Radiother Oncol 2001; 61: 233–46
- Jensen K, Grau C. Morbidity after Ipsilateral radiotherapy for oropharyngeal cancer. 2006 (submitted).
- Jensen K, Jensen AB, Grau C. A cross sectional quality of life study of 116 recurrence free head and neck cancer patients. The first use of EORTC H&N35 in Danish. Acta Oncol 2006; 45: 28–37
- Braam PM, Roesink JM, Moerland MA, Raaijmakers CP, Schipper M, Terhaard CH. Long-term parotid gland function after radiotherapy. Int J Radiat Oncol Biol Phys 2005; 62: 659–64
- Rademaker AW, Vonesh EF, Logemann JA, Pauloski BR, Liu D, Lazarus CL, et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: A 12-month follow-up study accounting for dropout. Head Neck 2003; 25: 1034–41
- Allenby A, Matthews J, Beresford J, McLachlan SA. The application of computer touch-screen technology in screening for psychosocial distress in an ambulatory oncology setting. Eur J Cancer Care (Engl) 2002; 11: 245–53
- Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol 2004; 22: 714–24
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76
- Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000; 88: 2164–71
- D'Antonio LL, Zimmerman GJ, Cella DF, Long SA. Quality of life and functional status measures in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 1996; 122: 482–7
- Hassan SJ, Weymuller EA, Jr. Assessment of quality of life in head and neck cancer patients. Head Neck 1993; 15: 485–96
- Terrell JE, Nanavati KA, Esclamado RM, Bishop JK, Bradford CR, Wolf GT. Head and neck cancer-specific quality of life: Instrument validation. Arch Otolaryngol Head Neck Surg 1997; 123: 1125–32
- Henson BS, Inglehart MR, Eisbruch A, Ship JA. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral Oncol 2001; 37: 84–93
- Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: The M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg 2001; 127: 870–6
- McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia 2002; 17: 97–114
- Taylor RJ, Chepeha JC, Teknos TN, Bradford CR, Sharma PK, Terrell JE, et al. Development and validation of the neck dissection impairment index: A quality of life measure. Arch Otolaryngol Head Neck Surg 2002; 128: 44–9