Abstract
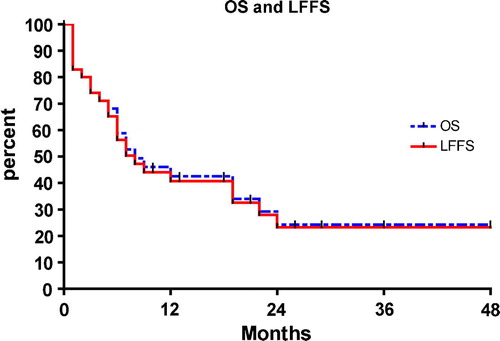
Background. Hodgkin lymphoma (HL)-related vanishing bile duct syndrome (VBDS) and idiopathic cholestasis (IC) are rare conditions that often lead to liver failure and death. The available literature consists primarily of case reports, resulting in little clarity as to the clinical course and ideal treatment for this disease. Material and methods. We performed a literature search from which we identified all published cases of HL-related VBDS or IC, and created a database of detailed presentation, treatment, and outcome information for all patients. Patient and disease factors were analyzed for an association with overall survival and liver failure-free survival. A case presentation introduces this analysis. Results. Thirty-seven cases of HL-related VBDS/IC were identified. Median follow-up was 7 months; 1-year OS and liver failure-free survival (LFFS) are 43% and 41%, respectively. Sixty-five percent of the patients died while 30% were alive with normal or near-normal stable liver function and no evidence of recurrent HL at last evaluation. Of the 20 patients without residual HL following therapy, 12 (60%) achieved liver failure-free survival. On univariate analysis, factors significantly associated with improved liver failure-free survival were stage I/II HL (p=0.02), a complete response of HL (p=0.0002), and delivery of radiotherapy (p<0.0001). Two patients received chemotherapy without radiation and survived with recovery of liver function. Discussion. HL-related VBDS/IC is potentially reversible and not uniformly fatal, with 30% of presenting patients demonstrating good lymphoma and liver outcomes after definitive therapy for HL. As a complete response of HL provides the only possibility of recovering liver function, patients with this disease should proceed to definitive treatment of HL as soon as feasible.
Vanishing bile duct syndrome (VBDS) is a unique clinical-pathologic entity with numerous etiologies. The syndrome consists primarily of severe icterus and hepatic laboratory abnormalities as a consequence of liver parenchyma that has undergone loss of normal bile ducts, hence the name of the syndrome. Interestingly, VBDS has an association with Hodgkin lymphoma (HL), an observation that has been described only in the last 14 years. The diagnosis of HL-related VBDS is likely part of a spectrum of hepatic disease that includes HL-related idiopathic cholestasis (IC), a disease identified nearly 50 years ago. HL-related VBDS/IC is essentially a diagnosis of exclusion, made by documentation of significant hepatic dysfunction along with distinct histological findings, in the absence of liver involvement of HL and other known causes of liver disease. The clinical signs and symptoms of VBDS/IC are typically the presenting complaints in this subpopulation of patients, the work-up for which usually leads to the diagnosis of HL.
Managing patients with HL-related VBDS/IC is challenging due to the complex medical management issues in the setting of minimal published data on interventions and outcomes, usually presented as single conflicting case reports. The hepatic damage associated with this disease is severe and often lethal. The rate of developing fulminate liver failure and death is unclear, and the role of medical therapy for this hepatic disease and liver transplantation is poorly documented. If liver failure is inevitable as some reports have suggested Citation[1–3], treatment of the associated HL may be of less clinical relevance. Alternatively, if HL is precipitating reversible hepatic injury, definitive therapy of the HL may prove to be the best therapy for the liver.
To gain a broader clinical perspective on this disease, we have identified and summarized all reported cases of HL-related VBDS/IC described in the literature, and analyzed the available information with a focus on clinical interventions and endpoints. We begin with a case report highlighting many of the issues discussed above. The patient who is described in the case presentation has provided informed consent for publication of his case.
Case presentation
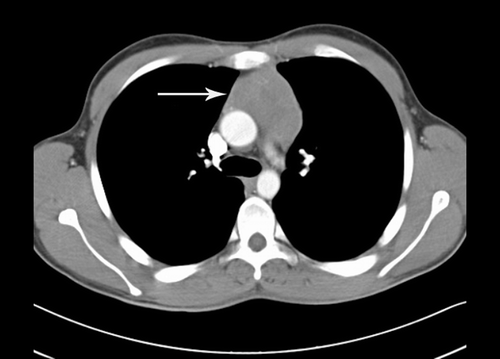
A 22 year-old previously healthy male presented in July 2004 with a 1-week history of jaundice, tea-colored urine, nausea, and vomiting. He was not taking any medications, and denied recent drug or alcohol use. Physical examination showed icterus and right-sided supraclavicular lymphadenopathy. Initial liver function tests (LFT's) showed an elevated total bilirubin of 26.0 mg/dL (normal: 0–1.0), direct bilirubin 16.0 mg/dL (normal: 0–0.4), indirect bilirubin 10.0 mg/dL (normal: 0–0.7), AST 134 U/L (normal: 0–47), ALT 189 U/L (normal 0–47), alkaline phosphatase 236 U/L (normal: 39–117), total protein 5.8 g/dL (normal: 6.4–8.3), and albumin 2.5 g/dL (normal: 3.4–5.0). Complete blood count, electrolytes, and kidney function were normal. Tests for HAV, HBV, HCV, HIV, and EBV were negative. Similarly, anti-nuclear antibody, anti-mitochondrial antibody, and anti-smooth muscle antibody were negative. A serum acetaminophen level was undetectable, urine toxicology was negative, and iron studies were normal. Erythrocyte sedimentation rate was 2 mm/hr (normal: 1–13). A chest x-ray revealed mediastinal lymphadenopathy and a contrast enhanced CT of the chest and abdomen showed an upper anterior mediastinal mass measuring 6.5 x 4.5 cm without hilar, axillary, or abdominal lymphadenopathy (). The liver was without evidence of biliary ductal dilatation, and the parenchyma appeared normal radiographically.
Figure 1. Axial CT scan of the chest with IV contrast showing a large anterior mediastinal soft-tissue mass measuring approximately 6.5 x 4.5 cm (depicted by white arrow).
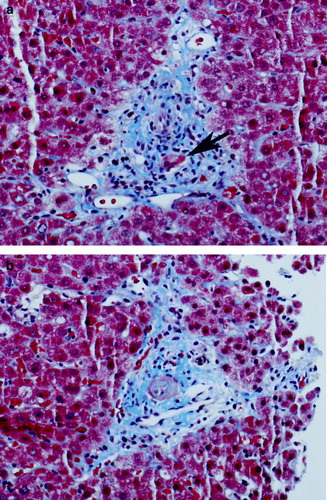
He was admitted and a fine needle aspirate of the right supraclavicular lymph node revealed large atypical cells consistent with either Hodgkin lymphoma or large-cell non-Hodgkin lymphoma. A bone marrow biopsy was performed and was negative for lymphoma. A percutaneous liver biopsy revealed that roughly two-thirds of the portal tracts contained bile ducts that were either obliterated or significantly injured (). Only mild patchy intrahepatic cholestasis was identified. No atypical cells suggestive of lymphoma or Reed-Sternberg cells were identified. Copper and iron stains were negative. The PAS-D stain showed no evidence of alpha-1 antitrypsin inclusions. The pathologic interpretation was bile duct loss with ongoing biliary injury consistent with VBDS.
Figure 2. Liver biopsy, photographs taken of Masson trichrome-stained slides at 100× original magnification. 2a) Bile duct injury: this photograph shows a complete portal triad demonstrating bile duct injury. The bile duct (arrow) shows distortion of the duct with loss of nuclei. In this injured duct, only two nuclei remain. The hepatic artery branch is just above the duct cross section. 2b) Bile duct loss: this photograph shows an artery that is similar in appearance to the artery in a (it is the structure in the middle of the portal tract in both cases). But, in contrast to the other photo, there is no bile duct cross-section in this portal tract, representing destruction of the bile duct.
Given the patient's severe liver dysfunction, he was initially treated with cyclophosphamide 800 mg/m2 on day 1 and dexamethasone 40 mg/day on days 1–4, and then continued on oral prednisone, 50 mg daily. He was discharged from the hospital and referred to the radiation oncology department as further chemotherapy was not considered feasible in light of his persistently abnormal liver function.
Upon evaluation in the radiation oncology department 24 days after presentation, it was noted that his supraclavicular lymphadenopathy had resolved and his total bilirubin decreased to 14.2 mg/dL. To definitively classify his lymphoma, he was referred for a Chamberlain procedure to biopsy his persistently enlarged mediastinal mass. Pathology revealed mononuclear atypical cells, with irregularly-shaped nuclei and single, prominent nucleoli that were positive for CD15 and CD30, and negative for CD20, CD3, and CD 45, consistent with nodular sclerosis Hodgkin lymphoma. Given the supraclavicular and mediastinal sites of involvement, he was staged as IIA.
He was treated with definitive radiotherapy alone using 3D conformal CT-based treatment planning, with treatment initiated 6 weeks after presentation. A standard mantle field was treated to 30.6 Gy with a boost of 9 Gy given to gross disease in standard fractionation (total dose 39.6 Gy). A para-aortic/spleen field was subsequently treated to 30.6 Gy (the liver was not within the treatment fields). Within 3 weeks of starting his mantle irradiation, his total bilirubin nearly normalized to 1.8 mg/dL. He was placed on azathioprine in September by his hepatologist, and began a prednisone taper.
Post-radiation CT and CT/PET scans showed a complete response to therapy. Prednisone was discontinued in 12/04, and azathioprine in 9/05. His last follow-up appointment was in 1/06 at which time he had no evidence of recurrent HL, his total bilirubin was 1.6 mg/dL and the remainder of his liver function tests was normal. Last contact with him was in 12/06, 29 months after presentation, at which time he was doing well without evidence of worsening liver function or recurrent HL. He has not had a repeat biopsy of his liver since his diagnosis.
Methods
We performed a literature search on www.pubmed.com to identify all case reports of HL-related IC or VBDS. Our searches included “idiopathic cholestasis and Hodgkin's”, and “vanishing bile duct syndrome and Hodgkin's.” From the articles identified, we retrieved any additional articles cited which referred to patients with HL-related IC or VBDS. Any publication in English or with an English translation was included. Detailed information regarding each published case was collected. Patients with lymphomatous infiltration of the liver or other likely explanations of liver dysfunction were excluded.
Patient, disease, and treatment factors for each published case were tabulated, including age, gender, stage, histology, B-symptoms, chemotherapy, radiation therapy, and steroid use. Staging was according to the American Joint Committee on Cancer Staging Manual, 6th edition, and determined from provided clinical information. Length of follow-up, HL status, liver function, and survival at last follow-up were recorded. Outcomes analyses were performed on the entire data set to determine overall survival (OS) and liver failure-free survival. As most articles did not provide specific laboratory values at last follow-up, we defined liver failure-free survival as alive without any clinical or laboratory evidence of liver failure.
Age (≤35 vs >35), gender, stage (I/II vs III/IV/relapse), HL histology (nodular sclerosing/lymphocyte predominant vs other), liver histology (VBDS vs IC), chemotherapy, radiation therapy, steroids, and HL response (complete response vs no complete response) were correlated to OS and liver failure-free survival using univariate analysis. Rates for OS and liver failure-free survival were generated using the Kaplan-Meier method and univariate comparisons were made using the log-rank test.
Results
The literature search identified 29 articles referring to 38 cases of HL-related VBDS or IC. Two articles referring to two cases of HL-related IC were not available in English Citation[4], Citation[5]. The remaining 27 articles describe 18 cases of HL-related VBDS, 16 cases of HL-related IC, one case of HL-related portal inflammation, and one case of HL-related idiopathic cholestatic hepatitis. With the inclusion of our patient, we report on a total of 37 cases described in the published literature. Information regarding duration of follow-up was not available in two cases; survival curves and univariate analysis therefore included a total of 35 cases. Median follow-up from diagnosis of IC/VBDS was 7 months (range 1–48).
Patient/disease characteristics
summarizes the patient, treatment, and outcome data for all 37 cases. The median age at diagnosis was 36.5 (range 3.5–72); 65% of patients were male. The stage of HL upon diagnosis of VBDS/IC was 21% stage I, 30% stage II, 27% stage III, 6% stage IV, and 15% with relapsed disease. B-symptoms were present in 54% of patients. HL histology was 54% nodular sclerosing, 30% mixed cellularity, 8% lymphocyte depleted, 4% lymphocyte predominant, and 4% Hodgkin's granuloma. Liver biopsy showed VBDS in 19 patients, IC in 16 patients, portal inflammation in one patient, and cholestatic hepatitis in one patient.
Table I. Summary of Reported Literature.
Clinical course
The Kaplan-Meier OS and liver failure-free survival curves for the entire group are shown in . One-year OS was 43% and liver failure-free survival (LFFS) was 41%. Overall, 65% (24/37) of patients died the majority (87%) from liver failure. However, 13 of the 37 patients were still alive at last follow-up, 12 who had stable LFT's with near-normal total bilirubin. Of these 12 patients with stable LFT's, 11 had no evidence of residual/recurrent lymphoma. Of the 20 patients without residual HL following therapy, 12 (60%) were alive with stable and normalized liver function. In total, 30% (11/37) of patients who presented with HL-related VBDS/IC were doing well with stable and near-normal LFT's and no evidence of recurrent HL at a median follow-up of 24 months.
Prognostic factors/treatment
Because the survival curves for OS and liver failure-free survival virtually overlap (), we selected liver failure-free survival as the endpoint for further analysis. Univariate analyses relating patient and treatment factors to liver failure-free survival are presented in . Kaplan-Meier survival curves for significant variables are presented in . The factors significantly associated with improved liver failure-free survival were stage I or II HL at diagnosis of IC/VBDS, radiation treatment, and a complete response to HL. Indeed, no patient had recovery of liver function without achieving a complete response of HL. Although chemotherapy was not predictive of liver failure-free survival, two patients were treated with definitive chemotherapy and had a complete response of HL, recovery of liver function, and were doing well at last follow-up.
Figure 4. Kaplan-Meier liver failure-free survival curves correlated to stage of Hodgkin lymphoma (4a), radiation treatment (4b), and HL response (4c). Abbreviations: LFFS = liver failure-free survival, HL = Hodgkin lymphoma, CR = complete response. P-values calculated using the log-rank test.
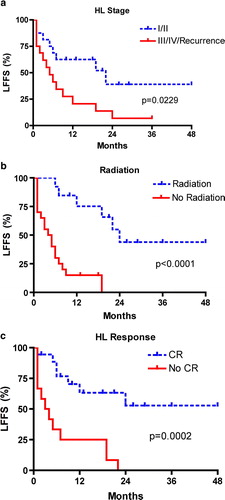
Table II. Univariate Analysis for Liver Failure–Free Survival.
Discussion
In this discussion, we summarize the identification of HL-related VBDS and IC, address whether HL-related VBDS and IC are the distinct entities or a spectrum of the same disease, describe the possible mechanisms of ductal destruction and cholestasis, summarize the clinical course, and suggest treatment strategies based on our experience and literature review.
Identification of HL-related VBDS and IC
It is well known that hepatic dysfunction may occur as a result of HL. Indeed, jaundice has been reported to be a symptom during the course of the disease in 3 – 13% of patients Citation[6], Citation[7]. Hepatic dysfunction most often occurs as a result of intrahepatic involvement of HL (61%) Citation[7], but also may be the result of extrahepatic bile duct obstruction, hemolysis, infectious hepatitis, or a toxic reaction to treatment such as chemotherapy or radiation therapy. In 1961, Bouroncle first described the phenomenon of HL-related IC in two patients with known HL but without lymphomatous involvement of the liver or precipitating causative factors for liver dysfunction Citation[7]. In the following 26 years, 10 more cases of HL-related IC were described in the literature Citation[8–14]. In 1993, Hubscher described three patients who were initially thought to have HL-related IC Citation[1]. Upon review of the pathology, it was noted that these patients actually had a paucity of small intrahepatic bile ducts, consistent with VBDS. Since that time, 16 cases of HL-related VBDS (including this case) Citation[2], Citation[3], Citation[15–25], 5 cases of HL-related IC Citation[26–29], and one case of HL-related idiopathic cholestatic hepatitis have been reported Citation[30].
Other documented causes of VBDS include developmental, immunological, infective, vascular, and chemical insults. Indeed, in some of the previous reports of HL-related VBDS or IC other potential hepatotoxic etiologies were present including ketoconazole Citation[1], medroxyprogesterone Citation[17], pregnancy Citation[10], myeloablative chemotherapy Citation[21], and erythromycin Citation[15]; leading one to question the exact etiology of liver injury in these cases. However, most of the reported cases of hepatic dysfunction occurred in the absence of any other identifiable cause, implicating HL as the inciting factor.
HL-related IC and VBDS: Same disease or different animals?
Whether HL-related IC and HL-related VBDS represent distinct entities or a spectrum of the same disease is unclear. Prior to Hubscher's article describing HL-related VBDS, most of the early reports of IC made no mention of a paucity of bile ducts. However, a few reports indicated that “canalicular plugging” was present, possibly representing early bile duct destruction Citation[8], Citation[9]. Since the publication of Hubscher's article, only five cases of HL-related IC have been described, while 16 cases of HL-related VBDS have been reported, suggesting that recognition of HL-related VBDS as a distinct pathologic entity has led to more prevalent designation of this specific diagnosis. VBDS was likely unrecognized in some patients prior to Hubscher's article, and instead diagnosed as IC, indicating these syndromes represent a spectrum of the same disease.
Interestingly, a case presented in 2002 by Liangpunsakul described a picture of HL-related idiopathic cholestatic hepatitis Citation[30]. Specific mention was made that no ductopenia was detected in the liver biopsy. They propose that this may represent an early form of ductal injury, which may eventually lead to VBDS. This is consistent with an early report of a patient with an initial biopsy which showed “subacute hepatitis” who, 1 year later, had another biopsy which showed “intracellular bile stasis and marked canalicular plugging,” possibly indicative of VBDS Citation[8]. Overall, it appears as though there may be an early stage of inflammatory activity which then leads to cholestasis and eventual loss of the intrahepatic bile ducts.
Mechanism of ductal destruction and cholestasis
The exact mechanism by which lymphoma is associated with or actually causes ductal destruction and cholestasis remains unknown. Various theories have been proposed, the most common being the emission of a cholestatic cytokine from the tumor cells. Piken could not identify an inciting endocrinologic etiology and speculated that there must be another mechanism Citation[11]. As previous publications have shown bile duct damage in relation to lymphomatous infiltration of the liver Citation[31], Citation[32], Hubscher posited that VBDS may be a lingering phenomenon left over from previous lymphomatous hepatic infiltration as two of his patients had received chemotherapy prior to the liver biopsy Citation[1]. He proposed that the lymphoma may have been present within the liver originally, but was eradicated by the chemotherapy, and was not present in the liver biopsies. However, 17 of the previously reported cases, including ours, had documentation of liver biopsies before the initiation of cytotoxic therapy, excluding lymphomatous infiltration of the liver as a possible inciting etiology. It seems that the most plausible explanation is that the lymphoma cells emit a cholestatic cytokine that is toxic to the liver, possibly in the form of an antibody, as in autoimmune causes of VBDS, or a small hepatotoxic molecule.
Clinical course
As this syndrome is rare, the clinical course has not been consistently or well defined. The literature presents mixed evidence as to whether HL-related VBDS/IC is reversible. In patients with VBDS of more common non-lymphomatous etiologies, once the bile ducts have disappeared, it is thought that they do not return Citation[33]. Similarly, multiple case reports of HL-related VBDS state that it is irreversible and patients should therefore be considered for liver transplantation Citation[1–3]. As shown in our analysis of all published cases, 32% (12/37) of patients with HL-related VBDS/IC were alive with stable and near-normal LFT's at last follow-up, indicating this disease is reversible in at least a subgroup of patients. Indeed, two patients actually had repeat post-therapy biopsies demonstrating regeneration of the intrahepatic bile ducts after complete remission of HL Citation[15], Citation[17].
A recent report by Barta suggests that HL-related IC is often reversible whereas HL-related VBDS is usually irreversible and fatal Citation[26]. However, our literature review and detailed analysis demonstrate no difference in clinical outcome between the VBDS and IC as 6/19 (32%) of the patients with HL-related VBDS, and 6/18 (33%) of the patients with HL-related IC, cholestatic hepatitis, or portal inflammation were alive with stable and near-normal LFT's at last follow-up. On univariate analysis, VBDS was not associated with worse liver failure-free survival (p = 0.59). While VBDS may represent a more advanced form of liver damage, it appears that both HL-related VBDS and IC are potentially reversible.
Treatment
As exemplified by , eradication of HL appears to be the most significant factor associated with an improved probability of liver function normalization and survival. This adds support to the theory that HL is somehow the direct or indirect cause of the hepatic injury. Although radiation is the only treatment modality that is significantly correlated with improved liver failure-free survival, this may be due to confounding factors, such as more advanced disease in patients who received chemotherapy only, and the inability to deliver curative chemotherapy in the setting of hepatic disease. Thus, we conclude that eradication of HL is of paramount importance to achieving liver failure-free survival.
Conventional treatment of early stage HL entails combination chemotherapy (often adriamycin, bleomycin, vinblastine, and dacarbazine) followed by involved field radiotherapy. As most patients presenting with HL-related VBDS/IC have significantly elevated bilirubin, it is often difficult to administer chemotherapy in effective doses and proven drug combinations. Patients with compromised liver function due to extrahepatic biliary obstruction by HL can be managed initially with chemotherapy regimens comprised of agents which are safe in the face of liver dysfunction; standard regimens can be instituted once liver function improves. This strategy may be possible in patients with HL-related VBDS/IC if prompt recovery of hepatic function occurs. However, recovery of liver function is often slow in patients with HL-related VBDS/IC, and radiation alone becomes the best treatment option. Fortunately, definitive radiotherapy with extended fields yields equivalent overall survival rates when compared with combined modality therapy in patients with favorable early stage HL Citation[34]. Patients with this syndrome should be referred promptly for consideration of radiotherapy, as definitive radiotherapy for early stage HL is not compromised in any way by the presence of hepatic dysfunction. Indeed, our patient underwent extended field radiotherapy and tolerated the treatment well. At 29 months since presentation, he is currently alive with near-normal LFT's, and without any evidence of recurrent HL.
Our analysis of all published cases in the literature demonstrates that HL-related VBDS/IC does not represent an irreversible form of liver damage, with approximately 1/3 of patients recovering hepatic function. Patients diagnosed with this combination of diseases should be referred for definitive therapy, preferably definitive radiotherapy if appropriate for disease stage, as definitive chemotherapy is usually not possible due to the degree of hepatic dysfunction. Only patients who experience remission of HL have any probability of hepatic function recovery and prolonged survival. As long-term follow-up in the literature is lacking, patients should be monitored closely for recurrence of HL and/or worsening hepatic function. Patients who have a complete response of HL, but continue to have liver failure should be considered for liver transplantation.
References
- Hubscher SC, Lumley MA, Elias E. Vanishing bile duct syndrome: A possible mechanism for intrahepatic cholestasis in Hodgkin's lymphoma. Hepatology 1993; 17: 70–7
- Rossini M, Lorand-Metze I, Oliveira G, De Souza C. Vanishing bile duct syndrome in Hodgkin's disease: Case report. Sao Paulo Med J 2000; 118: 154–7
- Gottrand F, Cullu F, Mazingue F, Nelken B, Lecomte-Houcke M, Farriaux JP. Intrahepatic cholestasis related to vanishing bile duct syndrome in Hodgkin's disease. J Pediatr Gastroenterol Nutr 1997; 24: 430–3
- Rodriguez-Gil FJ, Rincon-Fuentes JP, Garcia-Perez B, Conesa-Pallares FJ, Vicente-Lopez JJ, Grau-Garcia FJ, et al. Idiopathic cholestasis associated with bloody diarrhea as the first manifestation of Hodgkin's lymphoma. Gastroeneterol Hepatol 2006; 29: 240–3
- Parcero SA, Rubio RE, Huberman ED. Idiopathic cholestasis in Hodgkin's disease. Acta Gastroenterol Latinoam 1980; 10: 283–9
- Levitan R, Diamond HD, Craver LF. Jaundice in Hodgkin's disease. Am J Med 1961; 30: 99–111
- Bouroncle B, Old J, Vazques A. Pathogenesis of jaundice in Hodgkin's disease. Arch Intern Med 1962; 110: 872–83
- Juniper K. Prolonged severe obstructive jaundice in Hodgkin's disease. Gastroenterology 1963; 44: 199–204
- Groth C, Hellstrom K, Hofvendahl S, Nordenstam H, Wengle B. Diagnosis of malignant lymphoma at laparotomy disclosing intrahepatic cholestasis. Acta Chir Scand 1972; 138: 186–9
- Perera D, Greene M, Fenster L. Cholestasis associated with extrabiliary Hodgkin's disease. Gastroenterology 1974; 67: 680–5
- Piken E, Abraham G, Hepner G. Investigation of a patient with Hodgkin's disease and cholestasis. Gastroenterology 1979; 77: 145–7
- Trewby PN, Portmann B, Brinkley DM, Williams R. Liver disease as presenting manifestation of Hodgkin's disease. Quart J Med 1979; 189: 137–50
- Lieberman D. Intrahepatic cholestasis due to Hodgkin's disease. J Clin Gastroenterol 1986; 8: 304–7
- Birrer M, Young R. Differential diagnosis of jaundice in lymphoma patients. Sem Liver Disease 1987; 7: 269–77
- Cordoba Iturriagagoitia A, Inarrairaegui Bastarrica M, Perez de Equiza E, Zosaya Urmeneta JM, Marinez-Penuela JM, Beloqui Perez R. Ductal regeneration in vanishing bile duct syndrome in Hodgkin's lymphoma. Gastroenterol Hepatol 2005; 28: 275–8
- Yusuf MA, Elias E, Hubscher SG. Jaundice caused by the vanishing bile duct syndrome in a child with hodgkin lymphoma. J Pediatr Hematol Oncol 2000; 22: 154–7
- Crosbie OM, Crown JP, Nolan NP, Murray R, Hegarty JE. Resolution of paraneoplastic bile duct paucity following successful treatment of Hodgkin's disease. Hepatology 1997; 26: 5–8
- de Medieros BC, Lacerda MA, Telles JE, da Silva JA, de Medieros CR. Cholestasis secondary to Hodgkin's disease: Report of 2 cases of vanishing bile duct syndrome. Haematologica 1998; 83: 1038–40
- Allory Y, Metreau J, Zafrani E. Paraneoplastic vanishing bile duct syndrome in a case of Hodgkin's disease. Ann Pathol 2000; 20: 52–5
- Ozkan A, Yoruk A, Celkan T, Apak J, Yildiz I, Ozbay G. The vanishing bile duct syndrome in a child with Hodgkin disease. Med Pediatr Oncol 2001; 36: 398–9
- Kormurcu S, Ozet A, Altundag MK, Arpaci F, Ozturk B, Celasun B, et al. Vanishing bile duct syndrome occurring after high-dose chemotherapy and autologous peripheral stem cell transplantation in a patient with Hodgkin's disease. Ann Hematol 2002; 81: 57–8
- Ripoll C, Carretero L, Sabin P, Alvarez E, Marrupe D, Banares R. Idiopathic cholestasis associated with progressive ductopenia in two patients with Hodgkin's disease. Gastroenterol Hepatol 2002; 25: 313–5
- Guliter S, Erdem O, Isik M, Yamac K, Uluoglu O. Cholestatic liver disease with ductopenia (vanishing bile duct syndrome) in Hodgkin's disease: Report of a case. Tumori 2004; 90: 517–20
- Han WS, Jung ES, Kim YH, et al. Spontaneous resolution of vanishing bile duct syndrome in Hodgkin's lymphoma. Korean J Hepatol 2005; 11: 164–8
- Schmitt A, Gilden DJ, Saint S, Moseley R. Empirically incorrect. N Engl J Med 2006; 354: 509–14
- Barta SK, Yahalom J, Shia J, Hamlin P. Idiopathic cholestasis as a paraneoplastic phenomenon in Hodgkin's lymphoma. Clin Lymphoma Myeloma 2006; 7: 77–82
- Warner AS, Whitcomb FF. Extrahepatic Hodgkin's disease and cholestasis. Am J Gastroenterol 1986; 8: 304–7
- Jansen PL, van der Lelie H. Intrahepatic cholestasis and biliary cirrhosis associated with extrahepatic Hodgkin's disease. Neth J Med 1994; 44: 99–102
- Yalcin S, Kars A, Sokmensuer C, Atahan L. Extrahepatic Hodgkin's disease with intrahepatic cholestasis: Report of two cases. Oncology 1999; 57: 83–5
- Liangpunsakul S, Kwo P, Koukoulis GK. Hodgkin's disease presenting as cholestatic hepatitis with prominent ductal injury. Eur J Gastroenterol Hepatol 2002; 14: 323–7
- Cavalli G, Casali AM, Lambertini F, Busachi C. Changes in the small biliary passages in the hepatic localization of Hodgkin's disease. Virchows Arch [A] 1979; 384: 295–306
- Lefkowitch JH, Falkow S, Whitlock RT. Hepatic Hodgkin's disease simulating cholestatic hepatitis with liver failure. Arch Pathol Lab Med 1985; 109: 424–6
- Sherlock S. The syndrome of disappearing intrahepatic bile ducts. Lancet 1987; 2: 493–6
- Noordijk EM, Carde P, Dupouy N, Hagenbeek A, Krol AD, Kluin-Nelemans JC, et al. Combined-modality therapy for clinical stage I or II Hodgkin's lymphoma: Long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006; 24: 3128–35