Abstract
Background. Colorectal cancer (CRC) cell lines displaying microsatellite instability (MSI) are resistant to 5-fluorouracil (5-FU) in vitro, which can be overcome by restoring DNA mismatch repair (MMR) competence. Thymidylate synthase (TS) is inhibited by 5-FU, being another potential mediator of therapeutic resistance to 5-FU. The clinical relevance of these observations remains unclear. Objective. We examined the expression of TS and two MMR proteins (hMLH1 and hMSH2) in advanced CRC patients, to determine a) their mutual relationship, b) association to therapeutic response and c) impact on disease outcome. Material and methods. Tumour samples from 73 patients CRC who were treated in advanced stage with either irinotecan alone or in combination with 5-FU/leucovorin, were analysed for expression of TS, hMLH1 and hMSH2 using immunohistochemistry (IHC). Results. TS expression was closely correlated with hMLH1 expression (negative-weak/moderate-strong) (p=0.0001). TS-MMR expression was significantly (p=0.029 for whole series; p=0.004 for the 5-FU treated cases) related to response to treatment; tumours with low levels of both TS and MMR responded better (n=14/27, 51.8%) than those with high TS and MMR (n=3/18, 16.6%). Patients with high TS-MMR expression had a significantly longer DFS (47 months vs. 9months, n=26) than those with low TS-MMR index (p=0.015), while the reverse was true concerning survival with metastases (WMS) (p=0.018) in all the patients (n=73). Conclusions. The present data suggest that MSI patients with low TS and deficient MMR demonstrate a significantly shorter DFS and longer WMS than patients with high expression of both markers, and they are also more likely to obtain the greatest benefit from 5-FU based chemotherapy.
Colorectal cancer (CRC) is the second most frequent cancer in Europe in 2004, responsible for 13% (376 400) of all incident cancer cases. It is also the second most frequent cause of cancer mortality in Europe, with 11.9% (203 700) annual deaths Citation[1]. In the early stages, CRC is often a curable disease, but the overall prognosis is determined by the extent of local and particularly metastatic tumour spread. However, disease outlook is relatively poor for advanced disease and thus is a significant cause of worldwide cancer-related mortality Citation[1].
Fluoropyrimidine metabolites form a covalent complex with thymidylate synthase (TS). Formation of this complex prevents biosynthesis of intracellular thymidylate, which is essential for DNA biosynthesis. TS over-expression is an independent prognostic factor in different human cancers. High TS levels in hepatic metastases and resection margins are independent predictors of disease progression and survival in patients with metastatic CRC Citation[2]. Similar results have been reported in other tumours e.g. gastric, Citation[3] cervical, Citation[4] ovarian and head and neck cancers, where TS positive tumours have shown significantly worse outcome as compared to TS negative tumours.
Most CRCs display a phenomenon termed genomic instability. There are apparently two forms of genomic instability, which probably reflect different genetic pathways of tumorigenesis, microsatellite instability (MSI) and chromosomal instability (CI) Citation[5]. MSI is observed at the nucleotide level, frequently resulting in deletions or insertions of a few nucleotides. The inherent instability of microsatellite loci is primarily due to changes in the number of repeats during DNA replication because of inefficiencies in the DNA mismatch repair (MMR) system. This system normally identifies and repairs errors that may occur during DNA replication.
In experimental systems CRCs displaying MSI are resistant to 5-FU, which can be reverted by restoring DNA mismatch repair (MMR) proficiency Citation[6]. TS is inhibited by 5-FU, being another potential mediator of therapeutic resistance to 5-FU. Despite this potential link, data on mutual interactions between TS and MMR and their prognostic significance in CRC are emerging only recently Citation[7–9]. The clinical significance of these markers needs further assessment.
In this study, we examined the expression of TS and two MMR proteins, hMLH1 and hMSH2, in advanced CRC patients, to determine their interrelationships as well as their influence on response to chemotherapy and impact on patient survival.
Patients and methods
Patients, treatment and follow-up
A series of 73 patients were diagnosed and treated for stage II, III, and IV CRC at the Department of Oncology and Radiotherapy, Turku University Hospital (TUH) and six other hospitals of the same hospital district, between January 1996 and August 2003. The key clinical characteristics of the patients are summarized in .
Table I. Characteristics of the patients and their tumours at diagnosis.
At the time of diagnosis, 12 patients had stage II, 14 had stage III and 47 had stage IV disease. When patients received metastases or inoperable local recurrence, they were entered into the chemotherapy protocol. In the protocol, patients received one of two treatment regimes; 18 received irinotecan alone and 55 received a combination of irinotecan, 5-fluorouracil (5-FU) and folinic acid (FA) as first line treatment for metastatic disease. Irinotecan (350 mg/m2) was administered as a 60–90 min intravenous infusion every 3 weeks. In the combination regimen, irinotecan (180–210 mg/m2) was administered as 60–90 min intravenous infusion and 5-FU (500 mg/m2, i.v. bolus) modulated with folinic acid (FA) (60 mg/m2, i.v. bolus). The 5-FU/FA administrations were repeated again on the following day. The cycle was repeated every 2 weeks Citation[10]. The mean duration of chemotherapy was 6.5 months (±3.6). Treatment was continued until disease progression, or occurrence of unacceptable toxicity
Fifteen of 73 (20.5%) tumours were located in the rectum. None of them have received neoadjuvant radiotherapy since this was not the practice of our clinic at the time the patients were enrolled in study.
The patients were followed-up until the end of January 2005; mean follow-up time from diagnosis was 32.6 months (±24.8 months). We used three endpoints to calculate the patient survival: a) disease-free survival (DFS) this was calculated in 26 patients with stage II, III at diagnosis; b) overall disease-specific survival (DSS), and c) survival with metastases (WMS). DFS is the time from diagnosis to the appearance of metastatic disease, and relevant only for those patients with radically operated stage II, III patients at the time of diagnosis (n = 26). DSS is the time from diagnosis to death or to the time point when last seen alive at the clinic and calculable for all patients in the study. WMS was calculated from the date of recording the appearance of disease recurrence/metastases at the clinical visit, until to death or to the time point when last seen alive.
The study was approved by the TUH Ethical Committee and was conducted in accordance with the Declaration of Helsinki. Samples were collected with the endorsement of the National Authority for Medico-legal Affairs.
Immunohistochemical detection of Thymidylate synthase (TS) and hMLH1 and hMSH2
Seventy-three formalin-fixed, paraffin-embedded samples of the primary tumours were obtained from 73 patients. Sections were cut serially at 5 µm for routine haematoxylin and eosin staining and for immunohistochemical analysis. An experienced pathologist confirmed all histological diagnoses.
TS expression was studied immunohistochemically using monoclonal antibody (Mouse Clone TS 106, Zymed Laboratories Inc) diluted in 1% bovine serum albumin/Tris-buffered saline (1:25). Expression of hMLH1 and hMSH2 was studied using antibodies against hMLH1 and hMSH2 (Mouse anti-MLH1, Clone 14 and Mouse anti-MSH2, Clone FE11, Zymed Laboratories Inc.) diluted in 1% bovine serum albumin/Tris-buffered saline (1:50 or 1:100 respectively). The Detection was performed using the streptavidin-biotin method (Vectastain ABC kit). Formalin-fixed paraffin embedded sections were de-paraffinised in xylene, rehydrated in graded alcohol, immersed in 0.01M citrate buffer (pH 6.0), heated in a domestic microwave oven at full power for 2×5 min and left in the buffer to cool to room temperature. The sections were incubated in 0.3% hydrogen peroxide for 20 min to block endogenous peroxidase activity. Incubation with the primary antibodies diluted in 1% bovine serum albumin/Tris-buffered saline, were carried out overnight in a humid chamber at 4°C. The following day the slides were washed and incubated first with the biotinylated secondary antibody (30 min, 20°C), then with avidin-biotin-peroxidase complex (30 min, 20°C). Positive staining was visualised with 3,3' diaminobenzidine (DAB) substrate solution and the sections were counterstained with Mayer's haematoxylin. As negative controls, slides were processed with the omission of the primary antibodies.
Evaluation of TS and hMLH1 and hMSH2 expression
Two observers (RB, HL) assessed the expressions of TS and (hMLH1 & hMSH2) the expression of TS was first screened for an overview of the general staining pattern. Four pictures of each slide covering most of the tumour area, were taken with a light microscope (4×magnification) connected to a camera and AnalySIS v 3.00 software (Soft Imaging System GmbH, Munster, Germany). The expression of the TS in the four pictures was analysed using Imaging Research Inc., St. Catharine's, Ontario, Canada), which detected the brown colour of the positively stained tumour cells and counted the area of those cells in pixels and also counted in pixels the total tumour area. The percentage of the positively stained tumour cells from the whole tumour area was counted and used in further analysis. This method of evaluating the TS expression was able to distinguish between the presence of many cells expressing low amounts and a few cells expressing high amounts of TS such that the percentage of the TS expression reflected total TS expression in the tumour, which may be more relevant biologically.
The expression of (hMLH1 and hMSH2) was first screened for an overview of the general staining pattern. Loss of expression was recorded when nuclear staining was absent from cancer cell nuclei but preserved in normal epithelium and stroma. Tumour expression was classified as weak, moderate or strong relative to the expression of normal epithelial and stromal cells present in that tumour section. Evaluation and scoring of the IHC slides was carried out without knowledge of the clinical data.
For statistical purposes, the expression profiles of each marker were treated as dichotomous variables, where tumours with negative or weak expression of either hMLH1 or hMSH2 were one category (reduced expression), and all those with moderate or strong expression were grouped into the second category (normal expression). For TS, we used median values as cut-off to build up the dichotomous variable of low- and high TS expression. In addition, combined TS-MMR indices were created, using the dichotomous MMR variables and TS variables (median cut-offs), resulting in four possible combinations of TS/MMR: low/low; low/high; high/low; and high/high. Finally, these were converted to a 3-class index as follows: class 1, TS/MMR, low/low; class 2, TS/MMR, low/high or high/low; and class 3, TS/MMR high/high.
Statistical analysis
Statistical analyses were performed using the SPSS® (SPSS, Inc., Chicago, USA) and STATA (Stata Corp., Texas, USA) software packages (SPSS for Windows, version 13.0.1 and STATA/SE 9.2). Frequency tables were analysed using the χ2 test, with likelihood ratio (LR) or Fisher's exact test being used to assess the significance of the correlation between categorical variables. Differences in the means of continuous variables were analysed using non-parametric tests (Mann-Whitney or Kruskal-Wallis tests) for 2- and multiple independent samples, respectively. ANOVA (analysis of variance) was only used for deriving the mean values of in each strata. Univariate survival (life-table) analysis for the outcome measure (disease-specific survival (DSS), disease-free survival (DFS), and survival with metastases (WMS) was based on Kaplan-Meier method, and the groups were compared with the log-rank (Mantel-Cox) test. In all tests, the values p < 0.05 were regarded statistically significant.
Results
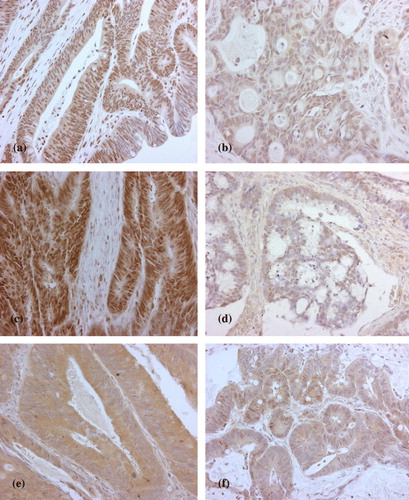
TS and MMR (hMLH1 and hMSH2) expressions were assessed using the median cut-off values, and the lesions were classified as low- and high expression categories (). High TS expression was directly related with hMLH1 (p = 0.0001) but not with hMSH2 (p = 0.174) expression, dichotomized as negative-weak and moderate-strong.
Figure 1. Examples of high (a), low (b) expression of hMLH1, and high (c), low (d) expression of hMSH2 (c, d). High /Low staining and TS staining (e, f) High/ Low (X20).
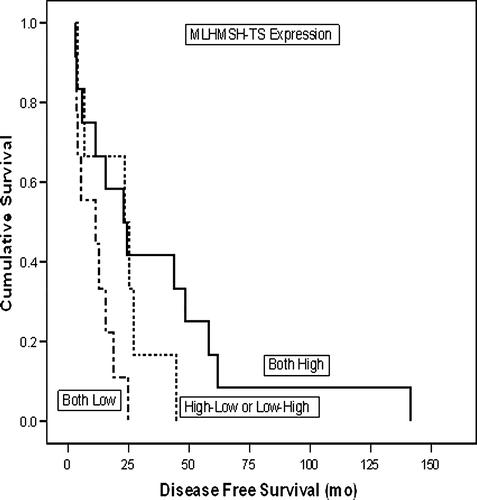
In the next step, the different combined TS-MMR indices were related to all clinical variables recorded, including the response to treatment and survival (DSS and DFS) rates. TS-MMR expression was significantly related to treatment response, as shown in . Using the 3-class indices, tumours with low levels of both TS and MMR responded better (52%), as compared with those showing high TS and MMR (17%) (p = 0.029). This same difference was even more accentuated among the 55 patients who received a combination of 5-FU plus FA with irinotecan; 63%, and 9%, respectively (p = 0.004).
Table II. TS-MMR expression* and response to treatment in the whole series and in patients treated with combined (5-FU) plus Irinotecan.
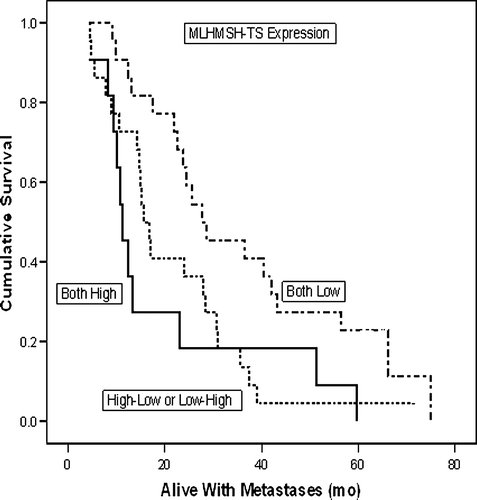
Patients with high TS-MMR expression presented with a significantly longer (47 months vs. 9 months) DFS than those with low TS-MMR index (p = 0.015). In univariate (Kaplan-Meier) survival analysis, this difference in DFS was also significant (n = 27; p = 0.028) (). In contrast, the patients with low TS-MMR index experienced a significantly (p = 0.019) longer survival with metastases (WMS) (). Accordingly, the median WMS was 28.1 months for those with low-low TS-MMR index, 16.2 months for those with high-low or low-high, and only 11.3 months for those who had both TS and MMR index high (p = 0.018, ANOVA). However, TS-MMR index was not significantly related to DSS in univariate analysis (p = 0.226) (data not shown in figures).
Figure 3. TS-MMR expression and survival with metastases (WMS) in univariate (Kaplan-Meier) analysis.
We have 26 patients with stage II, III, 11 who did not receive adjuvant and 15 who did receive. There was NOT a significant difference in WMS between the two groups: 20.8 months for those who received adjuvant and 33.2 months for those who did NOT receive adjuvant. p = 0.142 (ANOVA), and p = 0.235 (Mann-Whitney).
TS and MMR expression was not significantly associated with any other clinico-pathological variables, including patient's age, sex, TNM status, Grade, Stage or carcinoembryonic antigen (CEA) levels.
Discussion
For locally advanced and metastatic CRC, fluoropyrimidine, 5-fluorouracil (5-FU) has been the standard cytostatic drug for the last 50 years, used as modulated by leucovorin and in combination with irinotecan or oxaliplatin. An increase in TS protein levels has been suggested to be an important mechanism of resistance to chemotherapy with fluoropyrimidines Citation[11]. TS has been suggested as a prognostic factor of survival in CRC Citation[12–16] and a predictor of response to 5-FU therapy Citation[12], Citation[13], Citation[17], Citation[18]
In a previous study, we reported that higher levels of TS were associated with shorter survival in CRC patients and with low response rate to therapy Citation[19]. The present study is a continuation of this work, by comparing TS expression with the expression of MLH1 and MSH2. Investigation of DNA MMR status in CRC is relevant for several reasons: loss of one of the DNA MMR genes leads to the rapid accumulation of mutations. In addition an increased risk of tumorigenesis for example colorectal cell lines deficient in MMR show higher accumulation of other mutations and deletions Citation[20], Citation[21]. Germline mutations in the MMR genes are known to result in hereditary non-polyposis colorectal cancer (HNPCC), and somatic alterations Citation[22] or hypermethylation Citation[23], Citation[24] in this group of genes can result in the MSI found in approximately 10–15% of CRCs Citation[25], Citation[26].
To our knowledge only three studies have been published on CRCs reporting the interrelation of TS and MMR markers Citation[7–9]. Sinicrope et al. studied a group of patients with Dukes’ B2 and C-CRC who received 5-FU as adjuvant therapy and reported that the tumours with high frequency of MSI had an earlier stage at presentation and showed a better stage-adjusted survival rates. They also showed that the MSI status and TS expression were unrelated, and was of no prognostic significance, implicating that TS levels cannot explain the therapeutic resistance to 5-FU reported in tumours with high frequency of MSI Citation[7]. The second study by Ricciardiello et al. examined a group of patients with Dukes’ B, C and D and reported that tumours with MSI and defective MMR system show high expression of TS, indicating that also the tumours with high levels of TS do respond to chemotherapeutic agents Citation[8]. Popat et al. showed no relationship between MMR status and TS in a group of patients with stage A, B and C Citation[9].
Importantly, our study shows, for the first time the relationship between these two key molecules in a group of patients with locally advanced or metastatic CRC receiving similar treatment. Other studies have only hypothesized their relationship with treatment, whereas with the clinical data available in this study we have been able to assess this relationship Citation[8]. In the present study, we calculated a variety of indices to measure this TS-MMR co-expression, and these were related to different clinical variables, response to treatment and tested as predictors of disease outcome (DSF, DSS and survival with metastases). Of interest is the observed direct relationship between TS and hMLH1, with low- vs. high levels of TS correlating to those of hMLH1, respectively (p = 0.0001). This is the first study to establish such a direct relationship between hMLH1 and TS. Previously, Ricciardiello et al. reported an inverse correlation between hMLH1 and TS Citation[8], whereas the other two published studies could not establish any relation between MMR and TS Citation[6], Citation[8].
Despite the clear-cut experimental data implicating that CRC cell lines displaying MSI are resistant to 5-FU in vitro, which can be overcome by restoring their MMR competence Citation[21], controversy exists as to the clinical significance of these observations, i.e., resistance/sensitivity of MMR deficient CRC tumours to 5-FU based therapies. Thus, Liang et al. reported increased survival in patients with stage IV disease and MSI in their tumours, treated with 5-FU and leucovorin, suggesting that this may be due to an increased chemosensitivity in MSI tumours Citation[27]. In contrast, Rosty et al. were unable to discriminate the response to therapy in advanced CRC according to their MSI status Citation[28]. In the adjuvant setting, the results are more uniform, however. Accordingly, Elsaleh et al. found that tumours with the MSI+ phenotype appeared to be more sensitive to fluoropyrimidine-based chemotherapy, showing improved survival Citation[29]. Similarly, Hemminki and co-workers reported that patients with MSI+ tumours who receive 5-FU-based adjuvant chemotherapy had an excellent prognosis Citation[30].
These clinical observations are seemingly contradictory to the in vitro data Citation[21], while implicating that CRCs with MSI are, in fact, more sensitive to 5-FU-based therapies than their MMR competent counterparts Citation[26], Citation[28]. Our present observations are in alignment with these recently reported clinical observations. Thus, we also showed that tumours with low expression of TS and MMR (=MSI + ) appeared to be more sensitive to chemotherapy (). Accordingly, of the 28 patients with tumours showing low TS and MMR expression, 14 (52%) demonstrated an objective treatment response, as compared with only 17% of those with high TS-MMR index. This difference was even more marked in the group of patients (n = 55) who received 5-FU containing therapy; 63% vs. 9% (p = 0.004). In the light of these data and other published reports Citation[26], Citation[28], it seems feasible to speculate that tumour cells’ ability to repair DNA damage (i.e., their MMR status) should influence on their response to chemotherapeutic agents that cause the DNA damage. One would expect that, if this capacity is well retained (i.e., high MMR index), the response to DNA damaging agents (like 5-FU) would be more modest than in cases where MMR fails (i.e., low MMR index). The latter should lead to accumulation of mutations that might increase the sensitivity of the tumour cells to cytotoxic agents, while in the former case; there may be reduced sensitivity to treatment also if the normal apoptotic response to accumulated DNA damage is dysregulated.
As to the patient survival, there was a significant correlation of TS-MMR expression with DFS, in that the patients with high TS-MMR index had a longer DFS (). No such effect was shown to DSS, which was not significantly different among the patients with low- and high TS-MMR indices. However, patients with low TS-MMR index (who responded well to treatment) seemed to obtain a significant advantage in their survival time with metastases, being 28.1 months for those with low-low TS-MMR index, 16.2 months for those with high-low or low-high, and only 11.3 months for those who had both TS and MMR index high (p = 0.018, ANOVA). Taken together, this suggests that the longer DFS among high TS-MMR index patients (with poor response to treatment), is compensated by their shorter survival with metastases, leading to equalised overall survival (DSS) times between the low- and high TS-MMR-index patients.
This failure to ascribe an impact on DSS to TS-MMR index does not invalidate, however, the practical implications of these observations, of which two are the most obvious ones. First, patients with high TS-MMR expression seem to have a significantly longer DFS than those with low TS-MMR index. Those with intermediate (Class 2) index, also have a DFS intermediate (18 months) between these two extremes. On the other hand, these high expressors seem to respond purely to the therapeutic regimes used. Second, patients with low TS-MMR index are likely to be good responders to chemotherapy, and obtain a short benefit of survival with their metastatic disease. This survival advantage does not seem to be long enough to compensate their shorter DFS and to bring a net gain in their overall survival.
To summarise, the present data suggest that patients with advanced and metastatic CRC whose tumours have low TS and deficient MMR demonstrate a significantly shorter DFS, as compared to patients with high expression of these markers. However, these patients with low TS and MMR deficient tumours are also more likely to obtain the greatest benefit from chemotherapy.
References
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005; 16: 481–8
- Gonen M, Hummer A, Zervoudakis A, Sullivan D, Fong Y, Banerjee D., et al. Thymidylate synthase expression in hepatic tumors is a predictor of survival and progression in patients with resectable metastatic colorectal cancer. J Clin Oncol 2003; 21: 406–12
- Suda Y, Kuwashima Y, Tanaka Y, Uchida K, Akazawa S. Immunohistochemical detection of thymidylate synthase in advanced gastric cancer: A prognostic indicator in patients undergoing gastrectomy followed by adjuvant chemotherapy with 5-fluoropyrimidines. Anticancer Res 1999; 19: 805–10
- Suzuki M, Tsukagoshi S, Saga Y, Ohwada M, Sato I. Enhanced expression of thymidylate synthase may be of prognostic importance in advanced cervical cancer. Oncology 1999; 57: 50–4
- Jones AM, Douglas EJ, Halford SE, Fiegler H, Gorman PA, Roylance RR., et al. Array-CGH analysis of microsatellite-stable, near-diploid bowel cancers and comparison with other types of colorectal carcinoma. Oncogene 2005; 24: 118–29
- Koi M, Umar A, Chauhan DP, Cherian SP, Carethers JM, Kunkel TA., et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N′-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res 1994; 54: 4308–12
- Sinicrope FA, Rego RL, Halling KC, Foster NR, Sargent DJ, La Plant B., et al. Thymidylate synthase expression in colon carcinomas with microsatellite instability. Clin Cancer Res 2006; 12: 2738–44
- Ricciardiello L, Ceccarelli C, Angiolini G, Pariali M, Chieco P, Paterini P., et al. High thymidylate synthase expression in colorectal cancer with microsatellite instability: Implications for chemotherapeutic strategies. Clin Cancer Res 2005; 11: 4234–40
- Popat S, Wort R, Houlston RS. Inter-relationship between microsatellite instability, thymidylate synthase expression, and p53 status in colorectal cancer: Implications for chemoresistance. BMC Cancer 2006; 6: 150
- Glimelius B, Ristamaki R, Kjaer M, Pfeiffer P, Toren JS, Tveit KM, et al. Irinotecan combined with bolus 5-fluorouracil and folinic acid Nordic schedule as first line therapy in advanced colorectal cancer. Ann Oncol 2002; 13: 1868–73
- Peters GJ, van der Wilt CL, van Groeningen CJ, Smid K, Meijer S, Pinedo HM. Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: Implications for treatment with fluorouracil. J Clin Oncol 1994; 12: 2035–42
- Johnston PG, Fisher ER, Rockette HE, Fisher B, Wolmark N, Drake JC, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol 1994; 12: 2640–7
- Lenz HJ, Hayashi K, Salonga D, Danenberg KD, Danenberg PV, Metzger R, et al. p53 point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: An analysis of response and survival. Clin Cancer Res 1998; 4: 1243–50
- Lenz HJ, Danenberg KD, Leichman CG, Florentine B, Johnston PG, Groshen S, et al. p53 and thymidylate synthase expression in untreated stage II colon cancer: Associations with recurrence, survival, and site. Clin Cancer Res 1998; 4: 1227–34
- Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Kato T, Fukushima M, et al. A new prognostic factor for colorectal carcinoma, thymidylate synthase, and its therapeutic significance. Cancer 1998; 82: 70–7
- Edler D, Hallstrom M, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H. Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin Cancer Res 2000; 6: 1378–84
- Johnston PG, Lenz HJ, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res 1995; 55: 1407–12
- Leichman L, Lenz HJ, Leichman CG, Groshen S, Danenberg K, Baranda J, et al. Quantitation of intratumoral thymidylate synthase expression predicts for resistance to protracted infusion of 5-fluorouracil and weekly leucovorin in disseminated colorectal cancers: Preliminary report from an ongoing trial. Eur J Cancer 1995; 31A: 1306–10
- Bendardaf R, Lamlum H, Elzagheid A, Ristamaki R, Pyrhonen S. Thymidylate synthase expression levels: A prognostic and predictive role in advanced colorectal cancer. Oncol Rep 2005; 14: 657–62
- Mori Y, Yin J, Rashid A, Leggett BA, Young J, Simms L, et al. Instabilotyping: Comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res 2001; 61: 6046–9
- Haydon AM, Jass JR. Emerging pathways in colorectal-cancer development. Lancet Oncol 2002; 3: 83–8
- Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, et al. Microsatellite instability in colorectal cancer: Different mutator phenotypes and the principal involvement of hMLH1. Cancer Res 1998; 58: 1713–8
- Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998; 58: 3455–60
- Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 1997; 57: 808–11
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature 1998; 396: 643–9
- Halford S, Sasieni P, Rowan A, Wasan H, Bodmer W, Talbot I, et al. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res 2002; 62: 53–7
- Liang JT, Huang KC, Lai HS, Lee PH, Cheng YM, Hsu HC, et al. High-frequency microsatellite instability predicts better chemosensitivity to high-dose 5-fluorouracil plus leucovorin chemotherapy for stage IV sporadic colorectal cancer after palliative bowel resection. Int J Cancer 2002; 101: 519–25
- Rosty C, Chazal M, Etienne MC, Letoublon C, Bourgeon A, Delpero JR, et al. Determination of microsatellite instability, p53 and K-RAS mutations in hepatic metastases from patients with colorectal cancer: Relationship with response to 5-fluorouracil and survival. Int J Cancer 2001; 95: 162–7
- Elsaleh H, Iacopetta B. Microsatellite instability is a predictive marker for survival benefit from adjuvant chemotherapy in a population-based series of stage III colorectal carcinoma. Clin Colorectal Cancer 2001; 1: 104–9
- Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000; 119: 921–8
- Sobin LH, Greene FL. TNM classification: clarification of number of regional lymph nodes for pNo. Cancer 2001; 92: 452