We report a rare case of capecitabine-induced oromandibular dystonia and a summary of the cases reported in the literature.
A 57-year-old Chinese male was diagnosed with a pT3N2 stenosing adenocarcinoma of the rectum in January 2007, for which he underwent a low anterior resection. During staging evaluation, he was found to have a subcentimetre segment 2 liver lesion which was confirmed to be a solitary liver metastasis on positron emission tomography (PET). The plan was to give him two cycles of “neoadjuvant” capecitabine and oxaliplatin given every 3 weeks before resection of the liver metastasis, followed by further chemotherapy and radiotherapy. He was started on cycle 1 of capecitabine 2500 mg/m2 (days 1–14) and oxaliplatin 130 mg/m2 (day 1) on February 9, 2007.
Nine days after consuming capecitabine he developed sudden onset of disability to talk and swallow. Clinical examination revealed he had dystonia of tongue and pharyngeal muscles as well as involuntary jaw clenching. He did not have any other focal neurological signs nor associated metabolic abnormalities. He did complain of fatigue and anorexia but did not have features of hand-foot syndrome, haematological nor gastrointestinal toxicities.
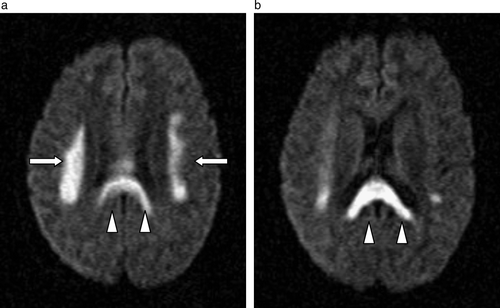
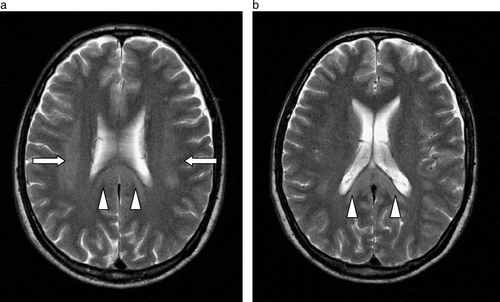
Computer tomography of his brain done on the same day showed no obvious abnormalities. However, a magnetic resonance imaging (MRI) scan was done which showed non-enhancing abnormalities with restricted diffusion involving bilateral corona radiate, the centrum semiovale and splenium of corpus collosum with scattered brainstem lesions noted ( and ) consistent with multifocal leukoencephalopathy.
Figure 2a & b. Axial diffusion- (a & b) and T2- (a & b) weighted images showing restricted diffusion and T2 signal hyperintensity involving the corona radiata (arrows) and splenium of the corpus callosum (arrowheads) in a symmetrical manner at initial presentation.
Capecitabine was discontinued on admission. A feeding tube had to be inserted as he had difficulty swallowing and he required a writing board to communicate. After 3 days, his symptoms completely resolved. Blood taken from the patient was analysed for TS 5′ gene polymorphisms and showed class 3 (3RG/3RC) genotype.
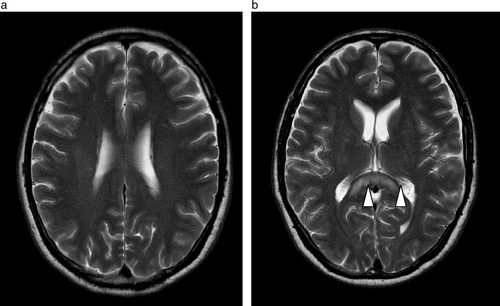
He was switched to raltitrexed and oxaliplatin as further therapy and no further neurological events occurred. A repeat MRI of his brain was done on March 20, 2007 (), which showed significant improvement in the periventricular leukoenphalopathy. He underwent an uneventful liver resection on April 17, 2007 and remains neurologically asymptomatic.
Figure 3a & b. Follow-up MR study performed a month later shows resolution of the previously noted T2W signal hyperintensity in the corona radiata with some residual T2W signal hyperintensity seen in the splenium of the corpus callosum (arrowheads).
Capecitabine is a proven oral chemotherapy agent in breast and colorectal cancer, which is increasingly used due to its ease of administration. Its antineoplastic activity is related to its final conversion to 5- fluoruracil (5FU). Side effects of capecitabine appear to be similar to those seen in 5FU treated patients, but with a more favourable profile Citation[1]. Capecitabine is a fluoropyrimidine carbonate which is rapidly absorbed and metabolized in the liver to 5′deoxy-5 flurocytidine (5′-DFCR) and then converted to 5′-deoxy-5-fluorouridine (5′-DFUR) by cytamine deaminase, The final step exploits higher concentrations of thymidine phosphorylase in tumour tissue which reduces 5′-DFUR to 5-fluotouracil (5-FU). Thus, the drug is selectively activated in the tumour and systemic toxicity is lessened Citation[2].
Here, we report a rare case of capecitabine-induced oromandibular dystonia without any other associated neurological signs, presenting 9 days after initiating treatment. Unlike 5FU, there have been few reports of capecitabine-induced central neurotoxicity and its incidence is estimated as less than 0.5% Citation[2]. Previous cases have reported symptoms of ataxia, cognitive changes, dysathria, epilepsy-like symptoms and even coma (see ) Citation[3–6] but not oromandibular dystonia alone. Onset of neurological symptoms occurred early within 3–7 days of initiating capecitabine therapy unlike neurotoxicity associated with 5FU which can occur later Citation[7]. Similar to previous reports, MRI of our patient's brain revealed white matter changes consistent with periventricular leukoencephalopathy and these changes resolved spontaneously together with the clinical symptoms, on termination of capecitabine therapy. These changes on imaging are also seen in 5FU-related leukoencephalopathy Citation[8], Citation[9]. Though, 5FU-encephalopathy has also been associated with an inflammatory leukoencephalopathy which has a delayed onset and recovery Citation[10].
Table I. Summary of case reports on capecitabine-induced multifocal leukoencephalopathy.
While the actual mechanistic reason for leukoencephalopathy in both capecitabine or 5FU use is poorly understood. Severe neurotoxicity from 5FU has been associated with dihydropyrimidine dehydrogenase deficiency (DPD) and it has been postulated that a DNA-directed or thymidylate synthase-based mechanism is the underlying event, thus making infusional thymidine a therapeutic option for patients with this toxicity Citation[11]. Small studies have also correlated DPD deficiency with capecitabine toxicity Citation[12] though not central neurotoxicity in particular. A trend toward a higher global toxicity grade 3 and 4 has been observed in patient's homozygous (class 4 genotype) for the TS 3RG allele compared with patient's heterozygous for the 3RG allele or not carrying the 3RG allele Citation[12]. Our patient was not assessed for DPD deficiency but was heterozygous for the 3RG allele.
Central toxicity in the absence of brain metastases may well indicate that capecitabine and its compounds can pass through the blood brain barrier (BBB). Capecitabine has also been shown to effectively cause regression of brain metastases Citation[13] though it is difficult to rule out the tumour-induced alterations in the BBB as a contributing factor. Nucleosides (like capecitabine metabolites 5′-DFCR and 5′ –DFUR) have been shown to be transported through the BBB by in vivo brain uptake index (BUI) and brain transfusion methods as well as in vitro isolated capillary and cultured endothelial cells Citation[14] and there is some evidence that 5FU itself may diffuse across the BBB too Citation[13]. The capecitabine metabolite, 5-DFUR, but not capecitabine itself or 5FU is transported via a substrate pyrimidine preferring transporter, hCNT1. Expression of this transporter promotes increased sensitivity of this prodrug and may thus determine cytoxicity of capecitabine Citation[15], Citation[16].
In summary, we present an unusual case report of capecitabine-induced oromandibular dystonia which had MRI and complete clinical resolution after discontinuation of the drug. As capecitabine usage increases, a better understanding of pharmacogenetics may shed light on mechanisms of capecitabine neurotoxicity. In the meantime, awareness among clinicians about this reversible chemotherapy adverse effect will avoid delay in diagnosis and management.
References
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Boyer M, Bugat R, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: A favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 2002; 13: 566–75
- Roche: Xeloda (capecitabine) Investigator's Brochure 2005.
- Couch LS, Groteluschen DL, Stewart JA, Mulkerin DL. Capecitabine-related neurotoxicity presenting as trismus. Clin Colorectal Cancer 2003; 3: 121–3
- Niemann B, Rochlitz C, Herrmann R, Pless M. Toxic encephalopathy induced by capecitabine. Oncology 2004; 66: 331–5
- Formica V, Leary A, Cunningham D, Chua YJ. 5-Fluorouracil can cross brain-blood barrier and cause encephalopathy: Should we expect the same from capecitabine? A case report on capecitabine-induced central neurotoxicity progressing to coma. Cancer Chemother Pharmacol 2006; 58: 276–8
- Videnovic A, Semenov I, Chua-Adajar R, Baddi L, Blumenthal DT, Beck AC, et al. Capecitabine-induced multifocal leukoencephalopathy: A report of five cases. Neurology 2005; 65: 1792–4
- Pirzada NA, Ali II, Dafer RM. Fluorouracil-induced neurotoxicity. Ann Pharmacother 2000; 34: 35–8
- Luppi G, Zoboli A, Barbieri F, Crisi G, Piccinini L, Silingardi V. Multifocal leukoencephalopathy associated with 5-fluorouracil and levamisole adjuvant therapy for colon cancer. A report of two cases and review of the literature. The INTACC. Intergruppo Nazionale Terpia Adiuvante Colon Carcinomal. Ann Oncol 1996; 7: 412–5
- Hook CC, Kimmel DW, Kvols LK, Scheithauer BW, Forsyth PA, Rubin J, et al. Multifocal inflammatory leukoencephalopathy with 5-fluorouracil and levamisole. Ann Neurol 1992; 31: 262–7
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 24-1999. Neurologic disorder in a 65-year-old man after treatment of colon cancer. N Eng J Med 1999;341:512–9.
- Takimoto CH, Lu ZH, Zhang R, Liang MD, Larson LV, Cantilena LR, et al. Severe neurotoxicity following 5-fluorouracil-based chemotherapy in a patient with dihydropyrimidine dehydrogenase deficiency. Clin Cancer Res 1996; 2: 477–81
- Largillier R, Etienne-Grimaldi MC, Formento JL, Ciccolini J, Nebbia JF, Ginot A, et al. Pharmacogenetics of capecitabine in advanced breast cancer patients. Clin Cancer Res 2006; 12: 5496–502
- Ekenel M, Hormigo AM, Peak S, DeAngelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol 2007 Jul 5; (Epub ahead of print).
- Bourke RS, et al. Kinetics of entry and distribution of 5-fluorouracil in cerebrospinal fluid and brain following intravenous injection in a primate. Cancer Res 1973; 33: 1735–46
- Mata JF, Garcia-Manteiga JM, Lostao MP, Fernandez-Veledo S, Guillen-Gomez E, Larrayoz IM, et al. Role of the human concentrative nucleoside transporter (hCNT1) in the cytotoxic action of 5[Prime]-deoxy-5-fluorouridine, an active intermediate metabolite of capecitabine, a novel oral anticancer drug. Mol Pharmacol 2001; 59: 1542–8
- Mackey JR, Yao SYM, Smith KM, Karpinski E, Baldwin SA, Cass CE, et al. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Natl Cancer Inst 1999; 91: 1876–81