Abstract
Introduction. Literature data show that mesothelioma cells can implant along the surgical pathway of invasive procedures such as thoracotomy and thoracoscopy. We investigated the use of hypofractionated radiotherapy for preventing such malignant seeding. Material and methods. Thirty-two consecutive patients diagnosed with pleural mesothelioma were included in the present retrospective study. All patients underwent surgery and/or thoracoscopy for diagnosis, staging or talc pleurodesis. They were treated with electron external beam radiation therapy (21 Gy in 3 fractions over 1 week), directed to the surgical pathway after the invasive procedure. After completion of radiation treatment, 20 of 32 patients (63%) underwent chemotherapy. Results. After a mean follow-up of 13.6 months (range 3–41) from the end of radiation therapy, no patient had tumour progression in the treated area. The treatment was well tolerated, as only erythema grade I (Radiation Therapy Oncology Group, RTOG, scale) was noted in 11 patients. Seventeen patients died of disease with local progression after a mean survival time of 12.6 months (range 3–27); thirteen patients are alive with disease after a mean follow-up of 13.9 months (range 4–41); two patients are alive without evidence of disease after a mean follow-up of 16.50 months (range 6–27). Discussion. The present study shows the efficacy and safety of local radiotherapy in preventing malignant seeding after thoracoscopy in patients with pleural mesothelioma although larger prospective trials are probably still needed to validate this treatment approach.
The diagnosis of pleural mesothelioma should always be confirmed by cytology or preferably pleural biopsy Citation[1], although the clinical history and radiological findings are usually typical. Disease stage at diagnosis is one of the most important prognostic factors and pathological post-surgical staging has been shown to be superior to computed tomography (CT) or magnetic resonance imaging (MRI) for accurate definition of tumour extension Citation[2], Citation[3]. Pleurectomy and extrapleural pneumonectomy are the most widely investigated surgical procedures Citation[4], Citation[5]. The paths of cytology or biopsy needles, chest drains, thoracoscopy trocars and surgical incision are common sites of malignant seeding after diagnostic and therapeutic procedures in patients with malignant pleural mesothelioma Citation[6–8]. Typically, seeding metastases consist of painful subcutaneous nodules varying from 1 to 4 cm in diameter. On the back, these lesions are particularly uncomfortable and often prevent patients from sleeping. In the presence of seeding metastases, surgical resection, when feasible, is the only effective procedure and radiotherapy is often ineffective Citation[7], Citation[9].
In the literature, the mean incidence of malignant seeding from pleural mesothelioma is 19% ranging from 2 to 51% Citation[7], Citation[10]. In the attempt of preventing that, radiotherapy has been used in a few retrospective and prospective clinical trials with non univocal results: tumour seeding ranged from 0 to 48% () Citation[7], Citation[11–15].
Table I. Details about prophylactic radiation therapy in malignant pleural mesothelioma from reported randomized and non-randomized studies and from current study
The purpose of this retrospective study was to assess the efficacy and safety of local hypofractionated radiotherapy in preventing malignant seeding in the path of drainage in patients with malignant pleural mesothelioma.
Materials and methods
Patients
From December 2001 to October 2006, 33 consecutive patients, 25 men, 8 women, mean age at diagnosis 64 years ranging from 44 to 84 years, diagnosed with malignant pleural mesothelioma and presenting a Karnofsky Performance Status (KPS) ≥70, were enrolled after obtaining the informed consent following the rules of our institution. One patient who already presented a painful subcutaneous recurrence before start of prophylactic treatment (30 days after thoracoscopy) was left out of the analysis. Twenty-one of 32 patients had undergone fine needle aspiration biopsy, 5/32 pleural cytology before thoracoscopy and 5/32 patients had directly undergone thoracoscopy. One patient with positive cytology had not received thoracoscopy but had undergone total pleurectomy (decortication). In 12 of 32 patients, talc pleurodesis was performed during thoracoscopy to prevent recurrent effusion.
Histological subtypes of mesothelioma were epithelial in 25 cases (78%), sarcomatoid in 6 cases (19%) and mixed in one case (3%). Disease staging was based on thoracoscopy findings, degree of extension and macroscopic appearance of lesions at chest CT-scan and abdominal ultrasonography. The International Mesothelioma Interest Group (IMIG) classification stage was as follows: Ia in 2 patient (6%), Ib in 3 patients (9%), stage II in 20 patients (63%), stage III in 4 patients (13%), and stage IV in 3 patients (9%). Surgical procedure, performed in 8/ 32 (25%) patients, consisted of radical pleurectomy in 6/32 patients (19%) and partial pleurectomy in 2/32 patients (6%).
Treatment
Radiotherapy was performed after healing of the surgical wounds typically 11 to 60 (median 37) days after thoracoscopy and 27 to 97 (median 45) days after pleurectomy.
Radiation treatment was performed by delivering a total dose of 21 Gy in 3 daily fractions (3×7 Gy) over 5 days to the thoracic wall. Electrons with energy of 12 MeV were delivered by a linear accelerator considering a reference isodose of 90%. All procedure scar sites (cytology, fine needle biopsy, drainage, thoracoscopy and pleurectomy/thoracotomy) were included in square/rectangular exposure fields ranging from 6×6 cm to 13×19 cm (median 100 cm2) ().
After completion of radiation treatment, 4/32 (12%) patients underwent 8 courses of single agent chemotherapy (pemetrexed, and 16/32 (50%) multiagent chemotherapy (pemetrexed and carboplatin in 12 cases, gemcitabine and oxaliplatin in 3 cases and tomudex and oxaliplatin and 5- fluorouracil and oxaliplatin in 1 case).
Follow-up was systematically performed by clinical examination every 3–4 months during the first 2 years and then every 6–8 months. Chest x-rays and/or CT scan were performed every 6–8.
Results
After a mean follow-up of 13.6 months (range 3–41) from the end of radiation therapy, no patient developed subcutaneous nodules in the treated area.
The treatment was well tolerated, as only temporary erythema grade I (RTOG scale) was noted in 11/32 patients (34%). No late effects were observed.
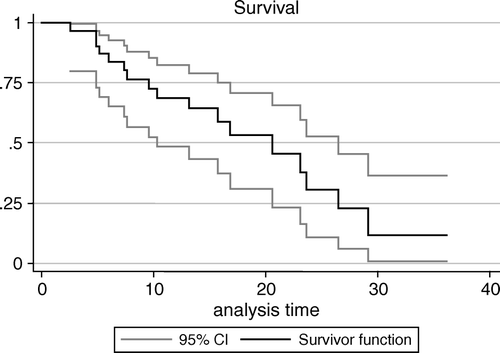
Actuarial overall survival calculated by Kaplan Meier method for the whole series was 68.9% (95% C.I. 51.8%–86%) at 1 year and 30.3% (95% C.I. 7.6%–53%) at 2 years (). Seventeen of 32 patients (53%) died of disease with local progression after a mean survival time of 12.6 months (range 3–27); thirteen patients (41%) are alive with disease after a mean follow-up of 13.9 months (range 4–41); two patients (6%), staged as II and Ia, are alive without evidence of disease after a mean follow-up of 16.5 months (6 and 27 respectively). The first patient with stage II disease had undergone only biopsy and pleurodesis before radiation therapy to the drain site and the second patient with stage Ia disease had received pleurectomy and chemotherapy.
Figure 2. Overall survival for the whole series of 32 patients estimated using the Kaplan-Meier method. Confidence intervals (C.I.) were computed according to Greenwood's formula for the standard error of cumulative survival. Analysis was carried on using SAS statistical software.
A minimal response (<50%) was observed in the patient with subcutaneous nodule already present at the time of radiation treatment and excluded from the statistical analysis. This patient died of disease 7 months after ending radiation treatment.
Discussion
Rationale for treating drain sites after invasive procedures
Currently the role of radiotherapy in the management of patients with malignant pleural mesothelioma is marginal; although postoperative treatment appears to limit in-field local failure, high-dose hemithoracic irradiation results in significant toxicity, including radiation-induced pulmonary fibrosis, radiation pneumonitis, and bronchopleural fistula, without any survival benefit Citation[8], Citation[16].
Since to date no combined modality treatment attempted was able to change the natural history of disease, patients with malignant pleural mesothelioma generally have a poor prognosis and most of them die of their disease within 5 years from diagnosis Citation[1], Citation[2], Citation[4–6], Citation[17]. In our study, only 6% of patients are alive without evidence of disease after a mean follow-up of 16.5 months. Therefore, it is important to consider not only treatments administered with a curative intent but also those given with a palliative intent in order to improve whenever possible the quality of life. Local radiation after invasive procedures, like surgery and thoracoscopy, may kill migrating cells before they are able to form a resistant colony. It is likely that single cells are more vulnerable in comparison to cellular clusters to irradiation. Moreover, irradiated tissue may be an unfavorable environment for malignant seeding resulting in a “tumor bed effect”. The mechanism usually proposed to explain the tumor bed effect are an alteration of the blood supply in the irradiated zone that results in a decrease in angiogenesis and thus in the supply of oxygen Citation[7].
The actual incidence of drain site seeding is still a source of some debate. In the literature, the mean incidence is 19% ranging from 2 to 51% Citation[7], Citation[10]. The original Boutin's trial reported a 40% seeding rate without radiation treatment whereas in both Bydder's and O'Rourke's trials this rate did not exceed 10% Citation[7], Citation[11], Citation[12]; Cellerin et al. reported an high incidence without radiotherapy (48%) in a retrospective series where the incidence of seeding in the treated arm was relatively high (21%) Citation[15]. Moreover, literature data do not clearly show whether some procedures have higher risk in malignant seeding than others; anyway, the incidence of tract metastases may be lower with computed tomography-guided biopsies than with thoracoscopies, as suggested by some authors Citation[10].
Efficacy of radiotherapy in preventing procedure tract metastases
A number of clinical trials studying the prophylactic use of RT have been conducted thus far (). The studies show different results in relation with clinical and technical aspects of the treatments. Hypofractionation and appropriate radiation energy seem to be important factors in order to maximize effectiveness. Moreover, inclusion of all scar sites in duly sized exposure zones (field selection) and timing of radiation therapy (prompt treatment) could be crucial too.
The rationale for using high dose per fraction relies on radiobiological findings showing that mesothelioma cells have a relatively low intrinsic radiosensitivity and a large shoulder in cell survival curves with a low alpha/beta ratio Citation[18]. Many different hypofractionated radiation treatment schedules have been tested: 8 or 10 Gy in 1 fraction, 16 Gy in 2 fractions, 18 Gy in 3 fractions, 20 Gy in 4 or 5 fractions and 21 Gy in 3 fractions over 1 week (equivalent to 45 Gy in 4,5 weeks). The latter is the most frequently reported in literature and appears to be the most effective Citation[7], Citation[11], Citation[13], Citation[14].
Likewise, high energies appear to be more effective (at least 10 MeV electrons) probably because they are able to cover more appropriately both depth and superficial tissues Citation[7], Citation[11], Citation[14].
In our prospective study, we delivered a dose of 21 Gy in 3 fractions over 1 week to the thoracic wall with 12 MeV electrons similarly to what proposed by Boutin et al. Citation[7] and to most of authors Citation[12–15]; our patients were treated 11 to 60 days after thoracoscopy and 27 to 97 days after pleurectomy and all procedure scar sites (cytology, needle biopsy, drainage and pleurectomy) were included in square/rectangular exposure zones ranging from 6×6 cm to 13×19 cm (median 100 cm2).
A delay in radiotherapy of more than 2 months was found to be associated with increased chest wall recurrence in a non-randomized study of 1983 by Boutin et al. (reported by the same author in 1995), so the authors underline the importance of a prompt treatment too Citation[7], Citation[12–15]. In our study we observed the onset of a seeding 30 days after thoracoscopy in the patient excluded from the present analysis, whereas no patients developed any recurrence in the treated areas and nearby tissues after a median follow up of 13.6 months (efficacy of 100%) in spite of they were treated 11 to 60 days (median 37) after thoracoscopy and 27 to 97 days (median 45) after pleurectomy. In this regard, we should underline that all our patients, except one, were treated within 60 days after surgical procedure and 61% of them received chemotherapy as well. By the way, a study by Pinto et al. Citation[19] did not report any case of malignant seeding after sole chemotherapy in a series of 54 patients. This fact may suggest an effect of chemotherapy on the incidence of drain site seeding. Based on these considerations, the ideal timing of radiotherapy and the eventual influence of using chemotherapy are still open matters deserving further investigation.
Dissonant responses come from two randomized trials: Bydder et al. Citation[11] and more recently O’ Rourke et al. Citation[12] didn't find any benefit about prophylactic radiation therapy on drain sites in patients with malignant pleural mesothelioma. Anyway, it is remarkable that Bydder examined a single 10 Gy treatment with electrons of relative lower energy (9 MeV) that maybe suboptimal, whereas in the O’ Rourke's trial the typical radiation field size (6 cm circle) was relatively small and 3/30 patients of the RT arm developed cutaneous seeding after subsequent invasive procedures not followed by a new prophylactic irradiation.
Most of the analyzed studies, including the present one, suggest a potential beneficial effect of prophylactic radiotherapy to intervention tracts ().
A recent survey performed on the radiotherapy practice of malignant pleural mesothelioma in The Netherlands and Belgium, came to a similar conclusion, stating that a consensus exists about prophylactic radiotherapy to intervention sites of malignant pleural mesothelioma using hypofractionated schedules Citation[20].
Adverse effects
The prophylactic radiotherapy technique described in the present study appears to be safe. Indeed, the treatment was well tolerated, as only erythema grade I (RTOG scale) was noted in 11 patients (34%) and no late effects were appreciable.
Although data on adverse effects were reported in only three randomized trials, they confirm the safety of treatment found in our retrospective study. Boutin et al. noted that tolerance to radiation therapy was excellent, with no patients exhibiting inflammation or edema but just showing some skin discoloration Citation[7]. Bydder et al. reported that no patients experienced Radiation Therapy Oncology Group (RTOG) or European Organization for Research and Treatment of Cancer (EORTC) grade 2–4 toxicities following treatment Citation[11]. O’ Rourke et al. observed 3/61 patients with persistent erithema/discolouration at radiotherapy site Citation[12].
Conclusion
Our study shows the efficacy and safety of local radiotherapy with appropriate energy, dose, and fractionation in preventing tract metastases after cytological study, needle, thoracoscopy, and pleurectomy in a consecutive series of patients with malignant mesothelioma.
Although no official guide-lines are available about local radiotherapy to be performed after invasive procedures for malignant pleural mesothelioma, it seems reasonable, considering our results and those of most of the analyzed studies, to propose such treatment approach in clinical practice.
Acknowledgements
The authors wish to thank Professor Corrado Magnani, chief of the Department of Epidemiology and Biostatistics of the University of Piemonte Orientale, for his contribution in the statistical analyses.
References
- Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–63
- Zucali PA, Giaccone G. Biology and management of malignant pleural mesothelioma. Eur J Cancer 2006; 42: 2706–14
- Heelan RT, Rusch VW, Begg CB, Panicek DM, Caravelli JF, Eisen C. Staging of malignant pleural mesothelioma: Comparison of CT and MR imaging. Am J Roentgenol 1999; 172: 1039–47
- Maggi G, Casadio C, Cianci R, Rena O, Ruffini E. Trimodality management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2001; 19: 346–50
- Sugarbaker DJ, Garcia JP, Richards WG, Harlpole DH, Jr, Healy-Baldini E, DeCamp MM, Jr, et al. Extrapleural pneumonectomy in the multimodality therapy of malignant pleural mesothelioma. Results in 120 consecutive patients. Ann Surg 1996; 224: 288–94
- Chapman E, Berenstein EG, Diéguez M, Ortiz Z. Radiotherapy for malignant pleural mesothelioma. Cochrane Database of Systematic Reviews 2006; Issue 3. Article number CD00388.
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995; 108: 754–8
- Ung YC, Yu E, Falkson C, Haynes AE, Stys-Norman D, Evans WK, et al. The role of radiation therapy in malignant pleural mesothelioma: A systematic review. Radiother Oncol 2006; 80: 13–8
- Treasure T, Sedrakyan A. Pleural mesothelioma: Little evidence, still time to do trials. Lancet 2004; 364(9440)1183–5
- Waite K, Gilligan D. The role of radiotherapy in the treatment of malignant pleural mesothelioma. Clin Oncol 2007; 19: 182–7
- Bydder S, Phillips M, Joseph DJ, Cameron F, Spry NA, DeMelker Y, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer 2004; 91: 9–10
- O'Rourke N, Curto Garcia J, Paul J, Hill J, Lawless C, McMenemin R, et al. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 2007; 84: 18–22
- Low EM, Khoury GG, Matthews AW, Neville E. Prevention of tumour seeding following thoracoscopy in mesothelioma by prophylactic radiotherapy. Clin Oncol 1995; 7: 317–8
- West SD, Foord T, Davies RJ. Needle-track metastases and prophylactic radiotherapy for mesothelioma. Respir Med 2006; 100: 1037–40
- Cellerin L, Garry P, Mahe MA, Chailleux E. Malignant pleural mesothelioma: Radiotherapy for the prevention of seeding nodules. Rev Mal Respir 2004; 21: 53–8
- Allen AM, Den R, Wong JS, Zurakowski D, Soto R, Janne PA, et al. Influence of radiotherapy technique and dose on patterns of failure for mesothelioma patients after extrapleural pneumectomy. Int J Radiat Oncol Biol Phys 2007; 68: 1366–74
- Ceresoli GL, Locati LD, Ferreri AJ, Cozzarini C, Passoni P, Melloni G, et al. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer 2001; 34: 279–87
- Hakkinen AM, Laasonen A, Linmainmaa K, Mattson K, Pyrhonen S. Radiosensitivity of mesothelioma cell lines. Acta Oncol 1996; 35: 451–6
- Pinto C, Marini A, De Pangher Manzini V, Benedetti G, Galetta D, Mazzanti P. Sequential chemotherapy with cisplatin/gemcitabine (CG) followed by mitoxantrone/metotrexate/mytomicin (MMM) in patients with malignant pleural mesothelioma. A multicenter Italian Phase II Study (SITMP I). Lung Cancer 2006; 52: 199–206
- De Ruysscher D, Slotman B. Treatment of intervention sites of malignant pleural mesothelioma with radiotherapy: A Dutch-Belgian survey. Radiother Oncol 2003; 68: 299–302