To the Editor
The high activity and low toxicity of exemestane, a third generation aromatase inhibitor, are well recognized Citation[1], Citation[2]. Its adverse effects are relatively mild and mainly related to anti-estrogenic effect Citation[1], Citation[2]. Compared to tamoxifen, only arthralgia and diarrhea occurred more frequently with exemestane Citation[1]. Neither was an impact of exemestane on lipid metabolism demonstrated Citation[3]. To our best knowledge, no cases of glucose tolerance deterioration or diabetes exacerbation associated with exemestane administration have been described. We apparently encountered such a situation in our patient treated with exemestane in a prospective clinical study.
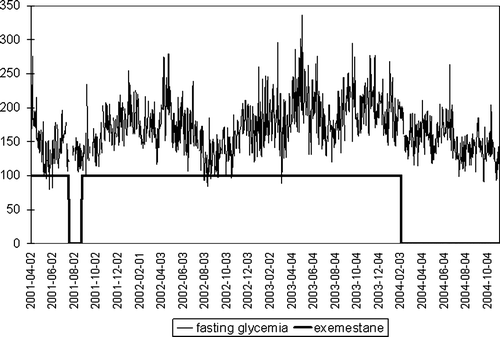
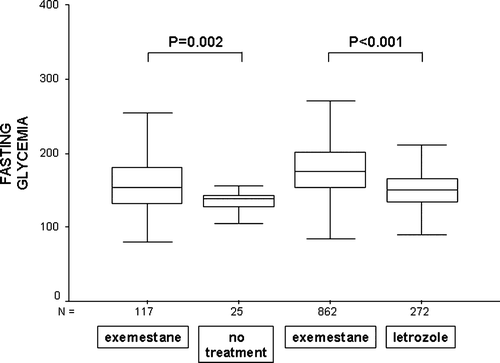
A 62-year-old female with a history of right mastectomy for invasive breast cancer in 1983 presented in January 2001 with inoperable chest wall recurrence. Her significant medical history included type 2 diabetes, well controlled with oral hypoglycemic agents. On February 2, 2001, she commenced treatment with exemestane (25 mg daily) within the EORTC 10951 phase III study comparing exemestane and tamoxifen in advanced breast cancer. Upon that, exacerbation of diabetes was observed (glycemia up to >400 mg/dl). After modification of treatment, her fasting glycemia stabilized at <180 mg/dl (period 1, median 153 mg/dl) ( and ). On July 17, 2001, exemestane administration was withdrawn for suspected progression. During the off-treatment period, her fasting glycemia did not exceed 160 mg/dl (period 2, median 139 mg/dl, p = 0.002) ( and ). There were no differences between postprandial glycemia “after breakfast” (p = 0.484) and “after lunch” (p = 0.303) in these two periods. Since additional examinations did not confirm disease progression, exemestane was reintroduced on August 23, 2001. Since then, glycemia began to rise steadily, eventually requiring the introduction of insulin. She continued on exemestane with almost complete tumor regression until February 4, 2004, with very unstable glycemia (period 3, median 176 mg/dl) ( and ), when, due to the unavailability of exemestane in Poland, she was switched to a non-steroidal aromatase inhibitor, letrozole. With this medication, her fasting glycemia lowered significantly (period 4, median 150 mg/dl, p < 0.001) ( and ). There was also a significant difference between these two periods for glycemia “after breakfast” (p < 0.001), “after lunch” (p = 0.05) and “before dinner” (p < 0.001). These changes were accompanied by a weight gain of 6 kg/6 weeks (most probably due to supraoptimal doses of insulin), requiring a decrease in hypoglycemic drugs. During the whole observation period no relevant changes in dietary plan, intake of calories or other recognized factors influencing the glycemia levels were present.
Figure 2. Median fasting glycemia levels (mg/ml) for periods on and off exemestane treatment (median values are indicated by middle lines, boxes represent internal quartiles, whiskers represent range).
Our results showed deterioration of diabetes related to exemestane. The relationship between increased androgen levels (secondary to low aromatase activity) and type 2 diabetes was described as early as in 1920s (“diabetes in woman with a beard”) Citation[4]. Recent studies elucidated its molecular mechanisms, linking single nucleotide polymorphisms (SNP) of genes involved in sex hormone metabolism (including CYP 19 gene, encoding for aromatase) with insulin sensitivity and risk of diabetes Citation[5]. Interestingly, a separate mechanism relating risk of diabetes to sex hormone levels has also been found in animal studies: estrogens were demonstrated to protect pancreatic ß-cells from apoptosis induced by oxidative stress, thus maintaining insulin production and preventing diabetes in mice Citation[6].
Importantly however, the phenomenon observed in our case was most probably unrelated to sex hormone levels, as opposite effects were observed during exemestane and letrozole treatments (otherwise producing similar hormone changes). Literature data on the effects of exemestane and non-steroidal aromatase inhibitors on lipid and bone metabolism suggest that despite their similarly potent estradiol lowering activities, these two groups have distinct features regarding their impact on at least some of estrogen dependent physiologic effects Citation[3].
It may be hypothesized that also insulin sensitivity is influenced by these two classes of aromatase inhibitors in a different way: exemestane is associated with diminished insulin sensitivity, whereas letrozole either has no effect or may even increase insulin sensitivity. This difference might be attributed to the steroidal vs the non-steroidal structure of each compound, respectively. Possibly, the androgenic structure of exemestane Citation[7] may be directly responsible for the diabetes-promoting action (independent from its effect on aromatase and estrogen levels), as demonstrated for other androgens. The elucidation of this phenomenon warrants further research.
Acknowledgements
We would like to thank Prof. Robert Paridaens, coordinator of the EORTC 10951 study for a kind permission to present this data. Dr Senkus-Konefka and Prof. Jassem received investigator fees from Pfizer.
References
- Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 2007; 369: 559–70
- Paridaens R, Dirix L, Lohrisch C, Beex L, Nooij M, Cameron D, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol 2003; 14: 1391–8
- Atalay G, Dirix L, Biganzoli L, Beex L, Nooij M, Cameron D, et al. The effect of exemestane on serum lipid profile in postmenopausal women with metastatic breast cancer: A companion study to EORTC Trial 10951, ‘Randomized phase II study in first line hormonal treatment for metastatic breast cancer with exemestane or tamoxifen in postmenopausal patients'. Ann Oncol 2004; 15: 211–7
- Achard MC, Thiers MJ. Le virilism pilaire et son association a l'insufficance glycolytique (Diabete des femmes a barbe). Bull Med Natl Med (Paris) 1921; 86: 51–66
- Lo JC, Zhao X, Scuteri A, Brockwell S, Sowers MR. The association of genetic polymorphisms in sex hormone biosynthesis and action with insulin sensitivity and diabetes mellitus in women at midlife. Am J Med 2006; 119(9 Suppl 1)S69–S78
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 2006; 103: 9232–7
- Zilembo N, Noberasco C, Bajetta E, Martinetti A, Mariani L, Orefice S, et al. Endocrinological and clinical evaluation of exemestane, a new steroidal aromatase inhibitor. Br J Cancer 1995; 72: 1007–12