Some decades ago, patients with a newly diagnosed rectal cancer were locally staged clinically, using the finger and a rectoscope. If the tumour was judged resectable, patients were operated upon whereas those judged not possible to resect were referred for preoperative radiotherapy. Delayed surgery was then performed if the tumour had diminished in size or was no longer clinically fixated by rectal palpation. In those days, 30–40% of the rectal cancers recurred locally even if surgery was successful. This often resulted in severe suffering prior to death. Five-year survival was less than 40% Citation[1–3]. Since then, most aspects of the care of rectal cancer patients have improved. The introduction of more precise surgery, total mesorectal excision (TME), and the use of pre- or postoperative radiotherapy or radiochemotherapy have contributed both to much lower local recurrence rates and improved survival. Presently, population-based studies can report local recurrence rates less than 10% and 5-years survival of 60% Citation[4–7]. Radiotherapy, alone or with chemotherapy is given not only to the 10–15% non-resectable cancers but also to many less advanced tumours since it lowers local recurrence rates and improves survival Citation[8], Citation[9]. Such comparably low levels of local recurrence rates can not be achieved if preoperative radiotherapy is only given to the most advanced T4 tumours Citation[10].
The availability of different treatment options, each having pros and cons (particularly concerning late adverse effects Citation[11]), has resulted in an increasing demand on better preoperative staging. Through the years, the possibilities to stage rectal cancer have also improved, although not introduced into clinical routine as rapidly as other aspects of care. Clinical staging, by evaluating fixation to the surrounding tissues is still used to identify locally advanced pelvic disease and to select patients for different preoperative treatments. The limitation of the clinical examination is obvious. In the case of large pelvic masses the mass effect of the tumour may cause fixation without corresponding tumour infiltration. There is also an obvious limitation of the clinical examination regarding estimation of tumour extent to different anatomical structures, some of them not reachable by the examining finger.
The different imaging techniques, used during the past decades to locally stage rectal cancer Citation[12], have to a various extent adopted pathological staging systems and used histopathology as a standard of reference to estimate sensitivity and specificity. In transrectal ultrasonography this had resulted in a uT-staging system, corresponding to the pathological pT-staging system. The sensitivity, specificity and limitations of different imaging techniques to stage early rectal cancer with pathology as a standard of reference is extensively documented, however, mostly in single-centre studies Citation[12]. In contrast, these parameters are not as well known for the locally advanced rectal cancers, since many of the surgical and pathological examinations of these tumours are performed after preoperative treatment with an effect on tumour stage Citation[13–15].
More recently, the circumferential resection margin (crm), the mesorectal fascia and the remaining lateral and inferior borders of the mesorectum have come into focus when staging rectal cancer. The ability to identify these landmarks by dedicated pelvic MR-imaging as well as predicting the degree of extramural tumour extension was the basis for the Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY) study Citation[16], Citation[17]. The positive experiences using a dedicated protocol for MR-imaging of rectal cancer in the study have resulted in ongoing discussions to stratify patients with rectal cancer based on MRI to be used for selection to preoperative treatment. In this issue of Acta Oncologica, Smith and Brown discuss preoperative staging of rectal cancer and present an algorithm for dividing the rectal cancers into three groups Citation[18]. The rectal tumours are then divided into those having no bad prognostic factors on MRI, neither for the risk of local or systemic failure (‘good group’, those having features on MRI suggesting increased risk for distant metastases (‘bad’) or those with features suggesting high risks for local recurrence and distant metastases (‘ugly’). The characteristics are depicted in figure 9 in Citation[18]. The new imaging aspects in this stratification are the extent of extramural extent of tumour in mm, the relation to the mesorectal fascia, the presence of more than four lymph node metastases and extramural vascular invasion. It is then important to recognise that beside extramural tumour extension and relation to the circumferential resection margin, the remaining prognostic factors evaluated by MRI are yet only reported by single centres.
The algorithm was used in a phase II trial, EXPERT, at the Royal Marsden Hospital, London, UK Citation[13], and is presently used in a multicentre randomised phase II study, the EXPERT-C protocol. In the trials, the ‘bad’ and ‘ugly’ groups are first given neo-adjuvant combination chemotherapy (capecitabine and oxaliplatin), then radiochemotherapy with capecitabine, surgery and postoperative chemotherapy, without or with cetuximab in the ongoing trial. The ‘good group’, not included in the trial, is operated upon, and postoperative therapy is given only if the pathological examination shows unfavourable signs. The neo-adjuvant chemotherapy is experimental but aimed at reducing the risk of systemic failure more efficiently than only postoperative chemotherapy can do. It is thus logical that the two groups included in the trial are characterised by high risks of systemic failure.
We have during the past years in Stockholm and Uppsala used a slightly different definition, adjusted to the therapy tradition in Sweden, presently emphasizing the local failure risk Citation[19]. Thus, a favourable, ‘good’ group has a low risk of failing locally, an intermediate ‘bad’ group has higher risk of failing locally and an advanced ‘ugly’ group has the highest risk of failing locally. The characteristics and the choice of therapy are shown in . The difference between our definition and the one suggested by Smith and Brown Citation[18] is in the definitions of the ‘bad’ and ‘ugly’ groups. We have not considered vascular invasion as a sign indicating increased risk of local failure and have therefore not incorporated that feature into the algorithm. It may be an important sign of systemic diseaseCitation[20], however, this has practically not yet had any influence on the choice of preoperative therapy. Besides the previously most locally advanced tumours corresponding to the ‘non-resectable T4s’, growing into adjacent organs, we also include the tumours growing adjacent to the mesorectal fascia (crm + ) to the advanced, ‘ugly’ group. The group at Royal Marsden Hospital, as most other international groups also tend to do, refer both the ‘bad’ and the ‘ugly’ tumours to the locally advanced cancers. Actually, the ‘non-resectable’ tumours, or the T4s growing into adjacent structures, have been excluded from all recent trials in these patients Citation[13], Citation[21–25]. They are also excluded from the ongoing EXPERT-C study, even if they have the greatest needs for more intensive, experimental therapy. Few studies have recently focused on the most advanced tumours Citation[14], Citation[26–28].
Figure 1. MRI-directed pre-operative evaluation practised presently in Uppsala and Stockholm, Sweden.
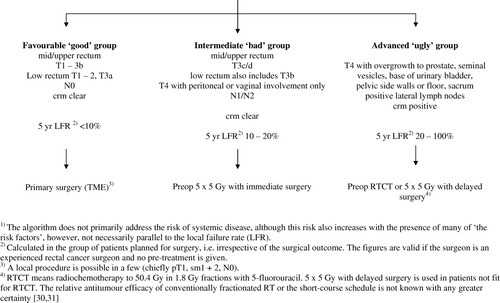
The use of imaging to identify patients regarded as having locally advanced rectal cancer has resulted in a shift towards including more and more patients as having this condition. The possible increase in number of patients regarded as having locally advanced rectal cancer due to ′staging drift′ must be taken into consideration when evaluating the effects of treatment of the disease. This is particularly relevant when conclusions are made from phase II studies exploring new treatments (many phase II studies published the past few years, none cited). It is possible that patient selection is more important than treatment efficacy Citation[29]. This ‘staging drift’ may be obscured if imaging as well as imaging interpretation is not strictly standardised using pre-study workshops and monitoring as described in the MERCURY-study.
In the future, there is a need to purify and more strictly standardise the term LARC. Using imaging, and in particular dedicated pelvic MR-imaging, this is possible provided that quality assurance, by work-shops and by involvement of radiologists in the multidisciplinary teams is established. Due to the different approaches of treatment offered by radiotherapy alone as to RTCT, it is reasonable to divide the locally advanced rectal cancers into level 1 or 2 depending on whether radiotherapy or radiochemotherapy should be used prior to surgery.
References
- Talbäck M, Stenbeck M, Rosén M, Glimelius B. Cancer survival in Sweden. Acta Oncol 2003; 42: 637–59
- Kapiteijn E, Marijnen CA, Colenbrander AC, Klein Kranenbarg E, Steup WH, van Krieken JH, et al. Local recurrence in patients with rectal cancer diagnosed between 1988 and 1992: A population-based study in the west Netherlands. Eur J Surg Oncol 1998; 24: 528–35
- Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007; 14: 447–54
- Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000; 356: 93–6
- Dahlberg M, Glimelius B, Påhlman L. Changing strategy for rectal cancer is associated with improved outcome. Br J Surg 1999; 86: 379–84
- Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol 2005; 31: 845–53
- Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002; 89: 1142–9
- Colorectal Cancer Collaborative Group. Adjuvant therapy for rectal cancer: A systematic overview of 8507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
- Glimelius B, Grönberg H, Järhult J, Wallgren A, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003; 42: 476–92
- Hansen MH, Kjaeve J, Revhaug A, Eriksen MT, Wibe A, Vonen B. Impact of radiotherapy on local recurrence of rectal cancer in Norway. Br J Surg 2007; 94: 113–8
- Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Late adverse effects of radiation therapy for rectal cancer–a systematic overview. Acta Oncol 2007; 46: 504–16
- Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: Local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging–a meta-analysis. Radiology 2004; 232: 773–83
- Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006; 24: 668–74
- Blomqvist L, Holm T, Nyren S, Svanstrom R, Ulvskog Y, Iselius L. MR imaging and computed tomography in patients with rectal tumours clinically judged as locally advanced. Clin Radiol 2002; 57: 211–8
- Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol 2007; 188: 442–51
- Brown G, Daniels IR. Preoperative staging of rectal cancer: The MERCURY research project. Recent Results Cancer Res 2005; 165: 58–74
- MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: Results of the MERCURY study. Radiology 2007;243:132–9.
- Smith N, Brown G. Preoperative staging in rectal cancer. Acta Oncol 2008;47: 20–31.
- Glimelius B. Rectal cancer irradiation. Long course, short course or something else?. Acta Oncol 2006; 45: 1013–7
- Smith NJ , Barbachano Y , Norman AR, Swift RI , Abulafi AM . Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2007; (in press).
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–23
- Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006; 24: 4620–5
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M, et al. Long-term results of a randomised trial comparing preoperative short-course radiotherapy vs preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–23
- Sebag-Montefiore D, Steele R, Quirke P, Grieve R, et al, for the NCRI colorectal cancer study group and CR07. Routine short course pre-op radiotherapy or selective post-op chemoradiotherapy for resectable rectal cancer? Preliminary results of the MRC CR07 randomised trial. J Clin Oncol 2006; Proc ASCO;24:Abstr 3511.
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- Braendengen M, Tveit KM, Berglund Å, Birkemeyer E, Frykholm G, Påhlman L, et al.A randomized phase III study (LARCS) comparing preoperative radiotherapy alone versus chemoradiotherapy in non-resectable rectal cancer. Eur J Cancer 2005; ECCO 13. Abstr 612.
- Frykholm GJ, Pahlman L, Glimelius B. Combined chemo- and radiotherapy vs. radiotherapy alone in the treatment of primary, nonresectable adenocarcinoma of the rectum. Int J Radiat Oncol Biol Phys 2001; 50: 427–34
- Pfeiffer P. High-dose radiotherapy and concurrent UFT plus l-leucovorin in locally advanced rectal cancer: A phase I trial. Acta Oncol 2005; 44: 224–9
- Glimelius B. Chemoradiotherapy for rectal cancer–is there an optimal combination?. Ann Oncol 2001; 12: 1039–45
- Courdi A. Fractionation sensitivity and equivalent doses. Commenting the Editorial by Glimelius. Acta Oncol 2007;46:395–6; author reply 396.
- Suwinski R, Wzietek I, Tarnawski R, Namysl-Kaletka A, Kryj M, Chmielarz A, et al. Moderately low alpha/beta ratio for rectal cancer may best explain the outcome of three fractionation schedules of preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2007; 69: 793–9
