Abstract
Background. Prediction of distant metastases is of paramount importance in the knowledge and management of breast cancer patients. The objective of this study was to assess conventional prognostic factors in a large database of patients with early breast cancer, including those with small tumors diagnosed through regional screening, to determine the risk of distant dissemination. Methods. The study included 4 797 patients of the Stockholm database who did not receive systemic adjuvant treatments. The main endpoint was metastasis free-interval. Individual risks of distant metastasis were estimated using the regression coefficients of the significant prognostic factors in Cox multivariate analyses. For each level of metastatic risk the pattern of failure was analyzed by a model assuming competing risks. Results. The three independent significant prognostic factors were histologic tumor size, number of involved axillary lymph nodes and progesterone receptor level. However, the latter factor added limited additional information of borderline clinical significance. Thus, subsequent estimations were done with a prognostic score taking into account only the former two most performant factors in the whole population. The risk of distant metastasis of observed values of tumor size categories fitted with published results of a series containing significantly larger tumors. A large variation of tumor size predicts 10-year distant metastasis risk ranging from below 10% up to 90%. Tumors of 10 mm or less had a 10-year metastatic risk of less than 10%. Conclusions. The results of this study are consistent with a linear effect of tumor size, within the range of data, on 10-year distant dissemination probabilities. Further refinement on prognostic value is needed for tumors of 15 mm or less.
Distant metastasis is the major cause of failure in the treatment of patients with early breast cancer. Even in patients with small tumors treated with breast conservation, the 10-year rate of distant metastasis is higher than 20% compared to 10% of local recurrences Citation[1]. Patients with local recurrence can be salvaged by a second local treatment Citation[2], but overt distant metastasis cannot be treated at the present time with a curative intent. The search for factors predicting the natural history of the disease has interested several investigators in an attempt to have a deeper knowledge of this pathology and to define different groups of patients according to the metastatic probabilities of their disease Citation[3–7]. The fact that prognostic factors for distant metastasis may be found is rather against the hypothesis that early breast cancer should always be considered as a systemic disease and suggests that medical interventions such as breast cancer screening, use of adequate loco-regional and systemic adjuvant treatments may improve the outcome. Indeed, it has been demonstrated that screening reduces the relative risk of breast cancer mortality by about 25% Citation[8] and that loco-regional control Citation[9] or effects of systemic adjuvant treatments Citation[10] have an effect of similar size in patients with early breast cancer by avoiding secondary dissemination Citation[11] or by eradicating micrometastases.
Koscielny et al. Citation[12] reported a series of 2 648 patients with resectable breast cancer treated at the Institut Gustave-Roussy (IGR). They showed that clinical tumor size is a good predictor of distant dissemination for tumors mainly comprised between 1.5 and 10 cm.
The purpose of this paper is to assess conventional prognostic factors such as age, histologic tumor size, lymph node status and hormonal receptors in a population of 4 797 early breast cancer patients, included in the Stockholm breast cancer database, to determine the risk of distant dissemination, to analyze patterns of recurrence in the different prognostic subsets, and to compare the results with those published with the IGR series. The advantage to study the Stockholm population is that most patients presented with smaller tumors as compared with the IGR series, as a result of screening programs.
Patients and methods
The study included 4 797 pre- and postmenopausal patients aged under 81 years with a resected invasive breast cancer treated in the Stockholm region between 1976 and 1988 who did not receive any systemic adjuvant treatment, other than breast radiotherapy in case of conservative surgery and lymph node irradiation in case of N+ axillary status. The exclusion of systemic treatments (chemotherapy, tamoxifen) was done to avoid confounding factors and facilitate the comparison with the IGR series Citation[12]. Patients with a past history of cancer were not eligible for the study. Surgery consisted of a modified radical mastectomy (3 967 patients, 83%) or of a partial mastectomy with axillary dissection followed by local radiotherapy (834 patients, 17%).
The median follow-up time, estimated by inverted Kaplan-Meier Citation[13], in this analysis was 7 years. Sixteen patients (0.4%) were lost to follow-up because of emigration.
Tumor size was measured in the surgical specimen and rated in millimeters by the pathologist. Axillary surgery consisted of a nodal dissection from level I and II. The median number of lymph nodes examined by the pathologist was 8.
Hormonal receptors were available in 3 199 patients. A part of the prognostic study was restricted to 3 080 patients with known progesterone receptors, histologic tumor size and number of histologically involved axillary lymph nodes. Steroid receptors analyses were performed on tumor samples using isoelectric focusing on polyacrylamide gel Citation[14]. The receptor values were normalized to DNA content as measured by Burton to avoid the influence of the large variations in extracellular protein Citation[15]. All assays were performed in one laboratory, a participant in the Swedish nationwide quality control program for hormone receptor laboratories sponsored by the Swedish Cancer Society. Patients with a receptor content of <0.05 fmol/µg DNA were considered as receptor-negative. The values in fmol/µg DNA can be approximately converted in fmol/mg tissue protein multiplying by 40.
Patients were regularly followed by clinical examination alone and yearly mammograms, other examinations being requested only in case of symptoms, at the oncologic clinics of the Stockholm region according to the following planned schedule: every 3 months in the first 2 years, every 6 months between 2 and 5 years and yearly after 5 years.
We planned to compare our results in terms of tumor extension and risk of distant metastasis with those published by the IGR Citation[12] in which patients did not receive either systemic treatments.
Statistical methodology
The main endpoint was metastasis-free interval (MFI). The only event taken into account was distant metastasis, ignoring other types of events. The MFI was calculated from the date of diagnosis to the date of distant recurrence, and in the absence of event, the censoring date was the date of last follow-up or death.
Univariate analyses using log-rank tests were performed to identify prognostic factors associated with MFI. Factors included in these analyses were: age, menopausal status, histologic tumor size, number of involved axillary lymph nodes, estrogen and progesterone receptors and treatment period (patients diagnosed in 1982 or before versus those diagnosed after). All factors considered significant at the p < 0.10 level by this method were included in a Cox multivariate analysis to identify the major independent prognostic factors. These independent factors were used to calculate a metastasis risk score (MRS) taking into account the regression coefficient (β value) of each factor Citation[16–18], with the formula MRS=(β value×category factor 1)+(β value×category factor 2), etc. The goal was to define an individual risk of distant metastasis for each included patient.
A model assuming competing risks Citation[19], Citation[20] was used to analyze the pattern of recurrence in the previously defined populations for the following events: local, distant metastasis, new primary malignancies and intercurrent death. Event-specific cumulative incidence curves were estimated from the decomposition of the event-free survival curves and incorporated into a computer program (COMPETE) developed at the Institut Gustave-Roussy. This approach has been described elsewhere Citation[21]; its main advantage is the absence of assumption of independence between tumor events such as local and distant recurrences and it permits a simultaneous and unequivocal description of all events intervening in the determination of failure patterns. In this context, event-free survival included the following events: local recurrence, distant metastasis, contralateral breast cancer, new primary malignancy and intercurrent death.
Results
Patient characteristics and univariate analysis
The patient characteristics and the results of the univariate and multivariate analyses are shown in . The following six variables had an effect on MFI: clinical T and clinical N of the TNM classification Citation[22], histologic tumor size, number of involved axillary lymph nodes, estrogen and progesterone receptors.
Table I. Patient characteristics and results of the univariate and multivariate analyses for 3 080 patients with known values of histologic tumor size, lymph node status and receptor status. Endpoint is distant metastasis.
Multivariate analyses
Including hormonal receptors
Because clinical T is correlated to histologic tumor size and clinical N to histologic lymph node status, these two clinical covariates were not included in the multivariate analyses. Among the four included variables, the following three were identified as having an independent unfavorable effect on MFI: increased histologic tumor size (p < 0.001), an increased number of involved lymph nodes (p < 0.001), and low values for progesterone receptors (p = 0.003). The results of the Cox analyses are shown in . Estrogen receptors did not add significant prognostic information (p = 0.092). Interactions between tumor size, lymph node status and estrogen receptors were not significant (p > 0.09).
Calculation of MRS: A first metastatic risk score (MRS1) was calculated taking into account the β values of each factor according to the following formula:Where: T size category = central value of each histologic tumor size category; involved lymph node category determined according to the number of involved axillary lymph nodes; PR category = progesterone receptor level. We also have done calculations with continuous tumor size values and this did not change estimations (data not shown).
For example, a patient with a tumor size of 25 mm, 7 involved lymph nodes and progesterone receptor equal to 0.2 fmol/µgDNA has a MRS1 of 2.12=(0.04×23)+(0.76×2)+(−0.32×1). The reference risk was that of a patient with a tumor size of 5 mm or less, with absence of axillary lymph node involvement and progesterone receptor level above 0.05 fmol/µDNA, for this patient the reference risk tumor was of −0.22.
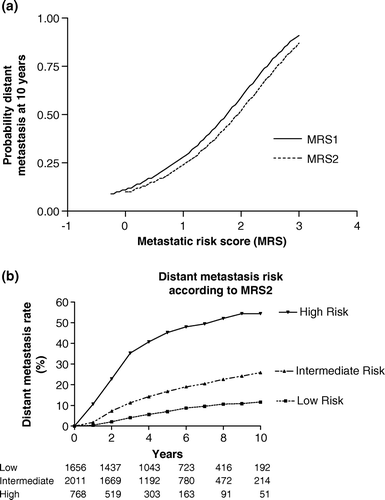
The observed values of MRS1 were plotted in a. The risk of distant dissemination at 10 years varied between 10 and 90%.
Figure 1a. Probability of distant metastasis at 10 years according to two metastasis risk scores (MRS) as a continuous variable, including (MRS1) and excluding progesterone receptors (MRS2). The other two factors considered were the histologic tumor size and the number of involved axillary lymph nodes. Figure 1b. Probability of distant metastasis at 10 years according to three groups of risk defined by two independent factors: histologic tumor size and number of involved lymph nodes. The metastasis risk groups were defined according to a score below 0.5 (low risk), between 0.5 and 1.5 (intermediate risk) and above 1.5 (high risk).
Excluding progesterone receptors
When progesterone receptors were used in the model, the study population was restricted to 3 080 patients because of missing values. To study a larger population (4 435 patients, ) only the two other significant factors were considered and a MRS2 was calculated as follows:
Table II. Results of the univariate and multivariate analyses for 4 435 patients with known values of histologic tumor size, and lymph node status. Endpoint is distant metastasis.
For the previous example, the MRS2 value was of 2.44 and for the patient with a reference risk tumor of 0.1.
The MRS2 values were plotted in a. The information given by the knowledge of the progesterone receptor level changed the estimation of the 10-year distant dissemination risk by 5% or less (only 2% for the patients with lower risk). Consequently, it seemed more convenient to continue the analyses using the MRS2 values which took into account a larger number of patients.
The MRS2 values were divided in three groups according to a 10-year metastatic risk of approximately 10, 25 and 50% as shown in b. Their prognostic value on metastasis was highly significant (p < 10−4). The actual overall 10-year metastasis rates were 12%, 26% and 54% for the low, intermediate and high risk groups, respectively. These risk groups represent 37%, 45% and 17% of the whole population.
Patterns of failure according to the risk of distant dissemination
The three groups defined by the MRS2 were analyzed by decomposing events determining event-free survival. In are shown for each risk group, event rates and relative risks in terms of first cause of failure within a competing risk approach and of total events (ignoring other events except death). Both local recurrence and distant metastasis rates were differently distributed among the three risk groups, and differences were significant regardless of the method used. There was no significant difference in the distribution of contralateral breast cancer. However, patients with a high risk of distant metastases had also a higher risk of new primary malignancy and intercurrent death. Indeed, for low, intermediate and high risk groups, the 3-year cumulative incidence of NPM was 1.7%, 2.0% and 2.9%, despite the similar 10-year rates shown in .
Table III. Ten-year cumulative incidence rates (first cause of failure and total events ) according to risk of distant dissemination in 4 435 patients with known values of histologic tumor size and lymph node status.
Modeling of distant dissemination according to histologic tumor size and lymph node status
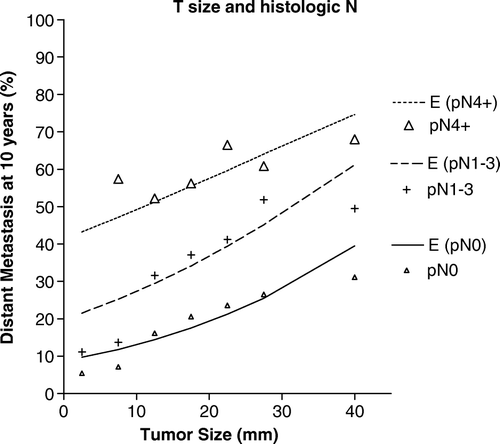
The 10-year distant metastasis rates were estimated from the coefficients of the Cox model and are shown in along with the observed values. The estimated lines allow to calculate the risk of metastatic dissemination taking into account the two independent factors.
Figure 2. Probability of distant metastasis at 10 years according to the histologic tumor size and the number of involved axillary lymph nodes. Lines represent the estimations obtained by a Cox model for the effect on each category and marks are the observed values for pN0 (o), pN1-3 (+) and pN4+ (Δ).
Comparison of the Stockholm data with the IGR published data
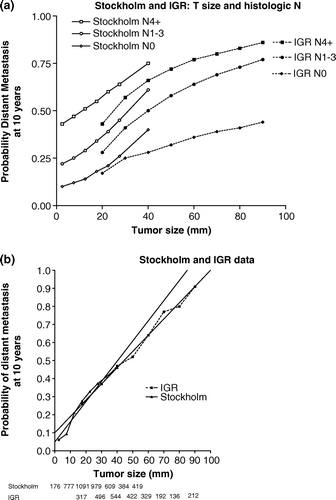
The present data was compared to that published by Koscielny et al. Citation[12] according to clinical tumor size and lymph node status (a). At each of the three lymph node levels the Stockholm series showed a moderately higher rate of distant dissemination – from 5 to 10% –when compared to the IGR series. In a second step, both series were compared only according to the observed values of tumor size categories, where an almost perfect fit is observed (b).
Figure 3a. Probability of distant metastasis for the Stockholm database – unfilled marks-compared with the IGR published data (12) – black marks – according to tumor size and lymph node involvement. Figure 3b. Overall results of 10-year distant metastasis rate for the Stockholm material –triangles – according to histological observed values of tumor size and for the IGR published data (12) – squares according to clinical tumor size. The linear fitting for the Stockholm series is r2=0.96, and for the IGR series r2=0.99; both highly significant (p < 0.0001). The numbers of patients in each group are given at the bottom.
Discussion
The present analysis showed that three independent prognostic factors influenced the metastasis-free interval in early breast cancer patients not receiving adjuvant chemotherapy or tamoxifen: histologic tumor size, number of involved axillary nodes and the progesterone receptor status. Our series is unusual because we selected patients who did not receive systemic therapies. For a similar treatment setting, Joensuu et al. Citation[23] studying a smaller series of node-positive breast cancer patients stressed the importance of an adequate loco-regional treatment.
Tumor size and number of involved axillary nodes are widely recognized prognostic factors and included in international treatment recommendations Citation[3], Citation[4], Citation[24–29]. It is possible that the statistical power of tumor size increases when carefully measured at the time of histologic examination as performed in the current series.
Hormonal receptors have been widely analyzed as prognostic factors, their significance has been variable according to different series Citation[4], Citation[5], Citation[30], Citation[31]. In the present study, with about 3 000 analyzed patients, estrogen receptors did not have an independent prognostic effect and progesterone receptors provided additional information of borderline clinical significance (a).
With the two strongest factors, i.e. histologic tumor size and lymph node involvement, it was possible to calculate a risk score permitting to determine different risk of metastasis at 10 years as shown in a. In the absence of information on progesterone receptors it was possible to evaluate a larger population of patients and to obtain a similar estimation as shown in the same figure. That means that only with two single and easily obtainable values it is possible to discriminate three populations with a different risk of dissemination (b). The use of scores as a continuous variable as shown in a allowed to define a wide variety of metastatic potential ranging from 10 to 90%. It would be difficult to obtain a better estimation by the use of new prognostic factors. However, as it can be seen in the model fit is not so good for tumors less than 10 mm with 0 to 3 involved axillary lymph nodes. The observed values showed a lower metastatic potential as compared to the model, with a 10-year metastasis rate lower than 15 and even 10%. This may be due to rounding errors, precision in measurements, or just random effect due to the small number of events in these smaller tumors. In this subgroup, new prognostic factors could be most useful, as it has been proposed by others Citation[6], Citation[7] to refine the prognosis indicators of this lower risk population for which the indication of systemic treatments is discussed. This need is of an increasing clinical importance as much more tumours are detected in an earlier stage through screening or a wider use of mammography for asymptomatic women Citation[32].
Koscielny and Tubiana published papers Citation[12], Citation[33] on the natural history of resectable breast cancer based on a population of about 3 000 patients treated at the Institut Gustave-Roussy (IGR) without systemic treatments and followed for more than 15 years. In summary, they found that the two most consistent prognostic factors of distant dissemination were tumor size and histologic lymph node involvement. They insisted on the discriminative value of tumor size which is an easily obtainable parameter. Most patients presented with tumors ranging from 15 to 90 mm. In the present series, most tumors ranged from 2 to 40 mm. To cover a wider spectrum of tumor size we plotted both series according to lymph node status in a. The plot showed that the pattern of distant metastases is similar in both series. However, at each of the three lymph node levels the Stockholm series showed a moderately higher rate of distant dissemination when compared to the IGR series. One possible explanation, other than well known difficulties in comparing different series, is that the median number of examined lymph nodes was 8 in the Stockholm material Citation[34] as compared to 15 in the IGR data. In this case a stage migration phenomenon could exist. To test this hypothesis we plotted in b overall results according to observed values of tumor size categories, the matching of results was much better, suggesting that results of a illustrates a typical example of stage migration Citation[35]. Overall results are compatible with a linear effect of tumor size on 10-year distant dissemination probabilities. Other authors have suggested that the percent of positive axillary nodes may also be an independent prognostic factor Citation[36].
The analysis of the pattern of failure showed that a high risk of distant metastasis was related to a higher risk of new primary malignancy (). However, this relationship was observed only in the first seven years. An explanatory hypothesis is that patients developing distant metastases had a higher probability of having a full assessment that could achieve earlier the diagnosis of a second cancer.
In this study, no histologic grade or correlated factors, such as nuclear DNA content, S-phase fraction, labeling or proliferation indexes, vascular invasion, or HER2 status, were available. However, these factors have not proved to add a higher discrimination in the categorization on node-negative patients Citation[2–4], Citation[37], Citation[38] as compared to the results shown in a-b, but they could refine the prognostic score value for lower risk patients, e.g. with tumors of 15 mm or less Citation[39–41]. For instance, Paik et al. Citation[6] showed recently that high histologic grade added prognostic information to the prediction of a genomic profiling in patients treated with tamoxifen.
Some recent reports Citation[6], Citation[7] raise the hope of new factors with a strong prognostic effect, but three conditions should still be fulfilled: i) results should be reproducible by other investigators and confronted in the frame of a multivariate analysis with conventional factors; ii) the new technique should be widely applicable; iii) use of large series of patients to avoid possibilities of misinterpretation related to statistical variations. The large statistical variability of results regarding genomic profiling as prognostic factors evaluated in rather small series has been recently stressed by Michiels et al. Citation[42]. The good prognostic information already obtained with the use of tumor size and lymph node status might explain difficulties with establishing new risk factors for distant dissemination. In addition, it is not sure that patients with borderline poorer prognostic factors will benefit more from systemic treatments. There is certainly a subsequent need to focus on predictive factors, i.e. those predicting the effect of a treatment intervention, such as that demonstrated for hormonal receptors (1), which should be analyzed in large randomized trials Citation[43] or in neo-adjuvant treatment series in which early treatment response is evaluated prospectively.
Acknowledgements
The study was supported by the King Gustav V Jubilee Fund, the Swedish Cancer Society, the Cancer Society of Stockholm and the General Motors Cancer Research Foundation. The authors are deeply indebted to Mrs. U. Johansson and T. Singnomklao for data managing, to Mrs. G. Feris for preparing the manuscript, and to the following investigators of the Stockholm Breast Cancer Group: Björn Cedermark, Lambert Skoog, Ulla Glas, Anders Somell, Tolle Theve, Nils Wilking, Juta Askergren, Marie-Louise Hjalmar, Tommy Fornander, Ulrik Ringborg and Jonas Bergh. They also thank Serge Koscielny for thoughtful advice.
References
- Sarrazin D, Dewar JD, Arriagada R, Benhamou S, Benhamou E, Lasser P, et al. Conservative management of breast cancer. Br J Surg 1986; 73: 604–6
- Lê MG, Arriagada R, Spielmann M, Guinebretière JM, Rochard F. Prognostic factors for death after an isolated local recurrence in patients with early-stage breast carcinoma. Cancer 2002; 94: 2813–20
- Arriagada R, Lê MG, Dunant A, Tubiana M, Contesso G. Twenty-five years of follow-up in operable breast cancer: Relationship between clinicopathological factors and death risk in each 5-year period. Cancer 2006; 106: 743–50
- Arriagada R, Rutqvist LE, Skoog L, Johansson H. Prognostic factors and natural history in lymph nodes-negative breast cancer patients. Breast Cancer Res Treat 1992; 21: 101–9
- Spyratos F, Martin PM, Hacène K, Romain S, Andrieu C, Ferrero-Pous M, et al. Multiparametric prognostic evaluation of biological factors in primary breast cancer. J Natl Cancer Inst 1992; 84: 1266–72
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351: 2817–26
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002; 347: 1999–2009
- Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet 2002; 359: 909–19
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087–106.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Arriagada R, Rutqvist LE, Mattsson A, Kramar A, Rotstein S. Adequate locoregional treatment for early breast cancer may prevent secondary dissemination. J Clin Oncol 1995; 13: 2869–78
- Koscielny S, Tubiana M, Lê MG, Valleron AJ, Mouriesse H, Contesso G, et al. Breast cancer, relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer 1984; 49: 709–15
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–6
- Wrange Ö, Nordenskjöld B, Gustafsson JA. Cytosol estradiol receptor in human mammary carcinoma. An assay based on isolectric focusing in polyacrylamide gel. Anal Biochem 1978; 85: 416–21
- Burton KA. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of DNA. Biochem J 1956; 62: 315–22
- Arriagada R, Mouriesse H, Sarrazin D, Clark RM, Deboer G. Radiotherapy alone in breast cancer. I. Analysis of tumor parameters, tumor dose and local control: The experience of the Gustave-Roussy Institute and The Princess Margaret Hospital. Int J Radiat Oncol Biol Phys 1985; 11: 1751–7
- Byar DP. Analysis of survival data: Cox and Weibull models with covariates. Statistics in Medical Research, V Miké, KE Stanley. J. Wiley, New York 1982; 365–401
- Christensen E. Multivariate survival analysis using Cox's regression model. Hepatology 1987; 7: 1346–58
- Gelman R, Gelber R, Henderson IC, Coleman CN, Harris JR. Improved methodology for analysing local and distant recurrence. J Clin Oncol 1990; 8: 548–55
- Kramar A, Pejovic MH, Chassagne DA. A method of analysis taking into account competing events: Application to the study of digestive complications following irradiation for cervical cancer. Stat Med 1987; 6: 785–94
- Arriagada R, Rutqvist LE, Kramar A, Johansson H. Competing risks determining event-free survival in early breast cancer. Br J Cancer 1992; 66: 951–7
- UICC. TNM: Classification of malignant tumors. 3rd ed. Geneva: 1979.
- Joensuu H, Pylkkänen L, Toikkanen S. Long-term survival in node-positive breast cancer treated by locoregional therapy alone. Br J Cancer 1998; 78: 795–9
- Rosen PP, Groshen S, Saigo PE, Kinne DW, Hellman S. Pathological prognostic factors in stage I (T1NoMo) and stage II (T1N1Mo) breast carcinoma: A study of 644 patients with median follow-up of 18 years. J Clin Oncol 1989; 7: 1239–51
- Sigurdsson H, Baldetorp B, Borg GA, Fernö M, Killander D, Olsson H. Indicators of prognosis in node-negative breast cancer. N Engl J Med 1990; 322: 1045–53
- Contesso G, Mouriesse H, Friedman S, Genin J, Sarrazin D, Rouesse J. The importance of histologic grade in long-term prognosis of breast cancer. A study of 1,010 patients, uniformly treated at the Institut Gustave-Roussy. J Clin Oncol 1987; 5: 1378–86
- Verschraegen C, Vinh-Hung V, Cserni G, Gordon R, Royce ME, Vlastos G, et al. Modeling the effect of tumor size in early breast cancer. Ann Surg 2005; 241: 309–18
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000; 124: 966–78
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005; 16: 1569–83
- Von Rosen A, Rutqvist LE, Carstensen J, Fallenius A, Skoog L, Auer G. Prognostic value of nuclear DNA content in breast cancer in relation to tumor size, nodal status, and estrogen receptor content. Breast Cancer Res Treat 1989; 13: 23–32
- McGuire WL, Tandon AK, Allred DC, Chamness C, Clark GM. How to use prognostic factors in axillary node-negative breast cancer patients. JNCI 1990; 82: 1006–15
- Hofvind S, Sørum R, Thoresen S. Incidence and tumor characteristics of breast cancer diagnosed before and after implementation of a population-based screening-program. Acta Oncol 2007; 1–7.
- Koscielny S, Tubiana M. The link between local recurrences and distant metastases in human breast cancer. Int J Radiat Oncol Biol Phys 1999; 43: 11–24
- Wilking N, Rutqvist LE, Carstensen J, Mattsson A, Skoog L, and participating members of the Stockholm Breast Cancer Study Group. Prognosis significance of axillary nodal status in primary breast cancer in relation to the number of resected nodes. Acta Oncol 1992;31:29–36.
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 1985; 312: 1604–8
- Atahan IL, Yildiz F, Ozygit G, Sari S, Gurkaynak M, Selek U, et al Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol 2007:1–7.
- Joensuu H, Toikkanen S, Klemi PJ. DNA Index and S-phase fraction and their combination as prognostic factors in operable ductal breast carcinoma. Cancer 1990; 66: 331–40
- Lonn U, Lonn S, Nilsson B, Stenkvist B. Breast cancer: Prognostic significance of c-erb B2 and Int-2 amplification compared with DNA ploidy, S-phase fraction, and conventional clinicopathological features. Breast Cancer Res Treat 1994; 29: 237–45
- Joensuu H, Isola J, Lundin M, Salminen T, Holli K, Kataja V, et al. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor as risk factors for distant recurrence in pT1N0M0 breast cancer: A nationwide population-based study. Clin Cancer Res 2003; 9: 923–30
- Joensuu H, Lehtimäki T, Holli K, Elomaa L, Turpeenniemi-Hujanen T, Kataja V, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA 2004; 292: 1064–73
- Lundin J, Lehtimäki T, Lundin M, Holli K, Elomaa L, Turpeenniemi-Hujanen T, et al. Generalisability of survival estimates for patients with breast cancer – A comparison across two population-based series. Eur J Cancer 2006; 42: 3228–35
- Michiels S, Koscielny S, Hill C. Prediction of cancer outcome with microarrays: A multiple random validation strategy. Lancet 2005; 365: 488–92
- Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24: 3726–34
