Abstract
Background. Trastuzumab is a monoclonal antibody that together with chemotherapy significantly improves time to progression and overall survival for metastatic breast cancer patients with tumours overexpressing HER2. The aim of this study was to analyse the cost-effectiveness of HER2 testing and trastuzumab in combination with chemotherapy compared with chemotherapy alone from a societal perspective in a Swedish setting. Material and methods. We used a Markov state transition model to simulate HER2 testing and subsequent treatment in a hypothetical cohort of 65 year old metastatic breast cancer patients. Outcomes included life-time costs, quality adjusted life years (QALY), and cost per QALY gained. Five different testing and treatment strategies were evaluated. Results. We estimated the cost per QALY gained to be about 485 000 SEK for the strategy of IHC testing for all patients, with FISH confirmation of 2+ and 3+, and trastuzumab and chemotherapy treatment for FISH positive patients. For the strategy of FISH testing for all patients, with trastuzumab and chemotherapy for FISH positive patients, we estimated the cost per QALY gained to about 561 000 SEK. The remaining testing and treatment strategies were dominated. Results were sensitive to changes in utilities, the risk of breast cancer related death, and test characteristics. Conclusion. Our analysis indicate that FISH testing for all patients with trastuzumab and chemotherapy treatment for FISH positive patients is a cost-effective treatment option from a societal perspective.
Breast cancer is the most common type of female cancer in Sweden, with approximately 7 000 new diagnosed cases and 1 500 deaths from the disease each year Citation[1], Citation[2]. Recent studies shows that breast cancer places a significant economic burden on the health care system, as well as being associated with considerable production losses arising from sick-leave and early retirement Citation[3].
Approximately 25 to 30% of breast cancers overexpress the human epidermal growth factor receptor-2 protein (HER2/neu), a product of the HER2 oncogene. Malignancies that overexpress HER2 are associated with a more aggressive disease course with a significant shortened overall survival Citation[4], Citation[5].
Trastuzumab is a monoclonal antibody that together with chemotherapy significantly improves time to progression and overall survival for metastatic breast cancer patients with tumours overexpressing HER2, compared with chemotherapy alone Citation[6], Citation[7].
The effectiveness of a targeted therapy such as traztuzumab depends on the identification of potentially receptive patients. Currently, the Swedish guidelines for identifying HER2 positive patients states that patients should be tested using immunohistochemical (IHC) tests, and confirming 2+ and 3+ patients with a fluorescence in situ hybridization (FISH) test Citation[8].
In 2005, trastuzumab sales was approximately 92 million SEK in 2005 in Sweden, which amounted to approximately 3% of total sales for drugs in the Anatomic Therapeutic Chemical (ATC) group L (antineoplastic and immunomodulating agents) Citation[9]. However, since the use of trastuzumab is associated with a substantial increase in the cost per patient treated (a treatment using 40 doses of 150 mg would add an extra 254 000 SEK in drug costs per patient Citation[10]), the use of trastuzumab can have a considerable impact on the budget of individual oncology clinics. Due to the limited resources available in the health care system, it is important that resources are used in a cost-effective manner.
Previous studies concerning the cost-effectiveness of traztuzumab for HER2 positive metastatic breast cancer patients have not reached consistent conclusions. The study by Hornberger et al. Citation[11] concluded that trastuzumab plus paclitaxel for first-line treatment of 3+ HER2-positive metastatic breast cancer was cost-effective, compared with paclitaxel alone. The National Institute for Health and Clinical Excellence (NICE) used this study when developing the guidance on the use of trastuzumab for treatment of advanced breast cancer Citation[12].
However, two subsequent studies have concluded that the cost-effectiveness of trastuzumab treatment is questionable. Both the study by Elkin et al. Citation[13] and the study by Norum et al. Citation[14] compared trastuzumab plus chemotherapy with chemotherapy alone and found that the incremental cost-effectiveness ratio (ICER) per QALY gained was in excess of what is normally considered cost-effective.
One reason for this inconsistency in the results of previous cost-effectiveness studies could be caused by how cross over from the chemotherapy alone arm to trastuzumab was handled in the different analyses. In the clinical study by Slamon et al. Citation[6], which was used in all of the three different cost-effectiveness studies to estimate the clinical benefit of trastuzumab, 66% of the patients initial assigned to the chemotherapy alone arm crossed over and received trastuzumab after disease progression. The study by Norum et al. Citation[14] did not adjust for this cross over, and thus actually compared trastuzumab + chemotherapy with chemotherapy alone and cross over to trastuzumab after disease progression, instead of comparing trastuzumab + chemotherapy with chemotherapy alone. Elkin et al. Citation[13] tried to adjust for the problem of cross over in their analysis by not calibrating the model to the overall survival of the chemotherapy alone arm. However, the impact of their adjustment on the final result is difficult to quantify. The study by Hornberger et al. Citation[11] used propensity scoring on unpublished trial data in order to obtain unbiased estimates of the time to death, thus adjusting for the problem of cross over.
Due to these inconsistent results of previous studies, further investigation of the cost-effectiveness of trastuzumab is important. With the publication of the clinical trial by Marty et al. Citation[7], published data on the overall survival of trastuzumab + chemotherapy as well as overall survival of chemotherapy alone (no cross over) is now available. Using published clinical trial data together with studies on the cost and quality of life of breast cancer patients, the aim of this study was to analyse the cost-effectiveness of HER2 testing and trastuzumab in combination with chemotherapy compared with chemotherapy alone from a societal perspective in a Swedish setting.
Material and methods
Cost effectiveness analysis
Cost-effectiveness analysis is a comparative analysis of both the costs and effects of two or more interventions. The effects are expressed in non-monetary units, such as life years (LY) gained or quality adjusted life years (QALY) gained. The incremental cost-effectiveness ratio (ICER) is defined as the ratio of the difference in cost to the difference in effect between the two comparators: ICER=(Ca–Cb)/(Ea–Eb), where Ci is the cost and Ei is the effect of intervention i. To properly calculate the ICER for an intervention, the intervention should be compared with the next most effective, non-dominated, intervention.
An intervention can be dominated for two reasons. The first reason is usually referred to as “simple dominance”, and arises when an intervention is less effective and more costly compared to an alternative intervention. The second alternative is when an interventions ICER is higher than that of the next more effective intervention. This is usually referred to as “extended dominance”. The concept of extended dominance is that the additional effect is produced at a higher marginal cost than necessary.
Model
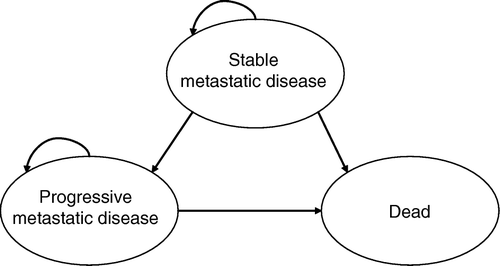
A model can be defined as a simplification of reality, and the goal of model design is to exclude irrelevant details while preserving the important characteristics of the system under study. As long as all relevant consequences of the treatment strategies are included, the result should not be dependent on the modelling technique used. The choice of modelling technique should therefore be based its ability to represent the available data in a simple and transparent way. We chose to construct a Markov state transition model, using monthly cycles, to evaluate the cost-effectiveness of five different strategies for identifying and treating HER2 positive patients ().
Table I. Test and treatment strategies.
The model consists of three different states: “Stable metastatic disease”, “Progressive metastatic disease”, and “Dead”. Patients start in the state “Stable metastatic disease” where they receive treatment according their HER2 test results. Patients can then transition to the state “Progressive metastatic disease” or the absorbing state “Dead”. Death due to breast cancer is only possible for patients in the state “Progressive metastatic disease”, while transition to “Dead” due to other causes is possible from any state. The reference patient was a 65 year old female with metastatic breast cancer, which is consistent with the age of metastatic breast cancer in clinical practise in Sweden. The model was created using DATA Pro (TreeAge, Williamstown, MA). The model is depicted in .
HER2 testing
IHC tests use antibodies to localise specific proteins in cells of a tissue section. Specimens are scored semi-quantitatively as to the intensity of membrane immunostaining on a four-point scale, with 0 indicating absence of staining, 1+ indicating the lowest level of detectable staining and/or nonhomogeneous weak staining, 2+ indicating moderate homogeneous membrane staining, and 3+ indicating intense homogeneous membrane staining Citation[15].
FISH is a technique that can be used to determine how many copies of a specific segment of DNA are present in a cell. It uses fluorescent DNA-probes, which bind only to those parts of the chromosome with which they show a high degree of sequence similarity. If the average HER2 gene copy number to chromosome 17 centromere copy number is greater than or equal to 2, or if the average number of HER2 gene copies per tumor cell nucleus is greater than or equal to 4, then the FISH test is considered positive for HER2 gene amplification Citation[16].
Studies seem to indicate that FISH is the best predictor of trastuzumab response Citation[17–19]. Because of this, we assumed that any benefit from trastuzumab treatment was derived in the presence of HER2 gene amplification. However, in Sweden, IHC tests are recommended for all patients, with FISH being used to confirm 2+ and 3+. The study by Elkin et al. Citation[13] contains a thorough overview of IHC test results compared to FISH test results. We have used their weighted average to estimate the IHC test results for FISH positive and FISH negative patients.
Table II. HER2 prevalence and HER2 testing.
Metastatic breast cancer progression and survival
Data on the time to progression (TTP) and the overall survival (OS) used in the base case scenario has been taken from the study by Marty et al. Citation[7]. In this study it was possible for the patients to cross over from receiving chemotherapy to receiving trastuzumab plus chemotherapy. Since we want to compare trastuzumab plus chemotherapy with chemotherapy alone, it is important to use OS of patients that has not crossed over. Using the estimates for patients that have crossed over would tend to underestimate the real difference in OS between the two treatment arms, and thus underestimate the benefit of trastuzumab plus chemotherapy. We therefore used the OS for patients that had received only chemotherapy without cross-over to trastuzumab to estimate the transition probabilities for patients receiving chemotherapy alone in our base case scenario.
Transition probabilities between the different disease states were estimated using median TTP and OS as well as Swedish life tables for the other cause mortality Citation[20].
Median TTP values from clinical trials were transformed into monthly transition probabilities between “Stable metastatic disease” and “Progressive metastatic disease” using the following formula:Using the transition probabilities derived from the median TTP and Swedish life tables, we iteratively adjusted the transition probabilities until the calibration model produced an overall survival that approximated the trial median OS. Transition probabilities are presented in .
Table III. Transition probabilities.
Cost
In the study by Marty et al. Citation[7] docetaxel was administered every 3 weeks using a dose of 100 mg/m2. Trastuzumab was administered as a 4 mg/kg loading dose followed by 2 mg/kg every week. We estimated the resulting drug cost using the average height and weight of Swedish women Citation[21] and drug prices obtained from the Swedish pharmaceutical reference book Citation[10]. A three week cycle of docetaxel was estimated to cost 15 406 SEK (20 541 SEK per month), and a one week cycle of trastuzumab was estimated to cost 5 653 SEK (22 612 SEK per month). Patients receiving docetaxel alone were assumed to have 1 outpatient visit every 3 weeks (1.33 outpatient visits per month) for the whole duration of their 1st line therapy. Patients receiving 1st line trastuzumab and docetaxel were assumed to have 1 outpatient visit every week (4 outpatient visits per month).
Patients ended their 1st line therapy if either the treatment duration was over (6 cycles of docetaxel or 40 cycles of trastuzumab) or if they had a progression. After patients had ended their 1st line therapy of either trastuzumab and docetaxel or docetaxel alone, it was assumed that their monthly outpatient drug cost was 1 868 SEK. This estimate was based on the expert opinion of an oncology specialist, and represents an estimation of the average cost of outpatient drugs given to metastatic breast cancer patients in Sweden (trastuzumab and docetaxel excluded).
The average outpatient cost after the end of 1st line therapy was based on a naturalistic study that estimated inpatient costs, outpatient costs, informal care costs, and indirect costs for breast cancer patients in Sweden Citation[22]. The average annual outpatient cost for a Swedish breast cancer patient with metastatic disease was estimated to 53 825 SEK. This outpatient cost was applied to all patients after they had ended their 1st line therapy.
The average annual inpatient costs and average annual informal care costs (care given by friends and family) for patients with metastatic disease was estimated to 34 938 SEK and 8 350 SEK respectively Citation[22]. These costs were applied to all patients from the start of start of 1st line therapy until death. No indirect costs were included in the model, since our reference patient was 65 years of age at the start of treatment.
Patients in the terminal phase of breast cancer are usually discharged from hospitals and receive palliative care either in their home or in a palliative care institution. This care is not included in the estimate of inpatient and outpatient cost, since it is financed by the municipality and takes place outside the hospitals. We included the cost of palliative care in our model as a one time cost for patients dying of breast cancer. The additional cost of palliative care was estimated to 63 244 SEK Citation[3], Citation[22].
In the study by Marty et al. Citation[7] 2 out of 92 patients (2.2%) in the trastuzumab and docetaxel arm experienced symptomatic congestive heart failure. We assumed that patients with symptomatic congestive heart failure would need to be investigated by a cardiologist. The additional cost of hospitalisation used to reflect this increase in adverse events for the trastuzumab and docetaxel patients were based on DRG group 127 (heart failure and chock), which had a unit cost of 32 049 SEK Citation[23], Citation[24].
The unit cost for the IHC test, FISH test and heart monitoring of patients treated with trastuzumab were gathered from an official price list of a large university hospital Citation[25]. The costs used in the model are given in .
Table IV. Costs and utility weights.
Costs are converted and inflated to year 2005 SEK: $1 = 7.48 SEK; €1 = 9.28 SEK and £1 = 13.58 SEK.
Quality of life
QALYs are estimated by multiplying the time spent in each health states with a weight reflecting the quality of life in that health state. A study by Lidgren et al. Citation[26] estimated the Health Related Quality of Life (HRQoL) in different breast cancer states from Swedish breast cancer patients. In our base case analysis, we used the average EQ-5D index value reported by patients with metastatic breast cancer, which was 0.685.
Results
In the base case analysis, the least costly and least effective strategy was chemotherapy alone for all patients (Strategy 1), which was associated with a cost of 331 668 SEK and yielded 1,28 QALY. The strategies of using IHC testing for all patients with trastuzumab and chemotherapy for 3+ patients (Strategy 2), was ruled out by extended dominance. IHC testing for all patients, with trastuzumab and chemotherapy for 2+ and 3+ patients (Strategy 3), was ruled out by simple dominance. The strategy of IHC testing for all patients with FISH confirmation of 2+ and 3+ and trastuzumab and chemotherapy for FISH positive patients (Strategy 4) was associated with a cost of 416 732 SEK and yielded 1,456 QALY, resulting in an ICER of 485 039 SEK/QALY when compared with Strategy 1, which was the lowest ICER of the different strategies evaluated. The most effective strategy in the base case was FISH testing for all patients, with trastuzumab and chemotherapy for FISH positive patients (Strategy 5). This strategy yielded 1,471 QALY and was associated with a cost of 425 174 SEK, giving it an ICER of 561 207 SEK/QALY when compared with Strategy 4. Results are presented in .
Table V. Base case cost-effectiveness analysis.
Using the cost per life-year gained as the unit of effect in the base case scenario, the ICER for Strategy 4 was estimated to 332 252 SEK/LY and the ICER for Strategy 5 was estimated to 384 427 SEK/LY.
Sensitivity analysis
An alternative scenario was evaluated were it was assumed that the monthly inpatient cost as well as the monthly outpatient cost after 1st line therapy differed between patients with stable metastatic disease and patients with progressive metastatic disease. It was also assumed that utility differed between patients with stable metastatic disease and patients with progressive metastatic disease. Data on cost and utilities were based on two Swedish studies Citation[22], Citation[26]. Based on these studies cost and utilities of patients who had at least 1 new distant recurrence more than 1 month after their first distant recurrence, was used as a proxy for patients with progressive metastatic disease. Cost and utilities for patients who did not have a distant recurrence, more than one month after their first distant recurrence, was used as a proxy for patients with stable metastatic disease.
For patients with progressive metastatic disease, annual inpatient costs was estimated to 94 339 SEK (7 862 SEK per month), annual outpatient costs were estimated to 60 772 SEK (5 064 SEK per month), and the utility was estimated to 0.661.
Patients with stable metastatic disease were estimated to have an annual inpatient cost of 23 811 SEK (1 984 SEK per month), an annual outpatient cost of 52 524 SEK (4 377 SEK per month), and the utility was estimated to 0.690.
Using these cost and utilities in the analysis, Strategy 4 was associated with a cost of 477 222 SEK and yielded 1,434 QALY, resulting in an ICER of 528 155 SEK/QALY. Strategy 5 was associated with a cost of 486 213 SEK and yielded 1,449 QALY, resulting in an ICER of 605 294 SEK/QALY. Just as in the base case scenario, Strategy 2 was ruled out by extended dominance and Strategy 3 was ruled out by simple dominance.
One-way sensitivity analyses were performed in order to test the robustness of the base case analysis, with 30% changes applied to key parameters. The results were relatively robust to these changes, and none of the changes (except for changes in the testing characteristics) altered the rank order of the different strategies or their dominance status. The most sensitive parameters were the utility scores, risk of breast cancer related death, and inpatient and outpatient costs. Strategy 2 and Strategy 3 were dominated in all of the different scenarios evaluated in the sensitivity analysis, and is therefore not reported in the tables and figures. The results of the one-way sensitivity analyses are given in .
Table VI. One-way sensitivity analyses, incremental cost per QALY gained.
We also performed two-way sensitivity analysis of impact of changes to sensitivity and specificity of the IHC test used to identify HER2 overexpression. Increases in the IHC test sensitivity and specificity had a small effect on the ICER of Strategy 4. However, changes in IHC test characteristics had a substantial impact on the ICER of Strategy 5. Results are presented in .
Table VII. HER2 testing two way sensitivity analyses, incremental cost per QALY gained.
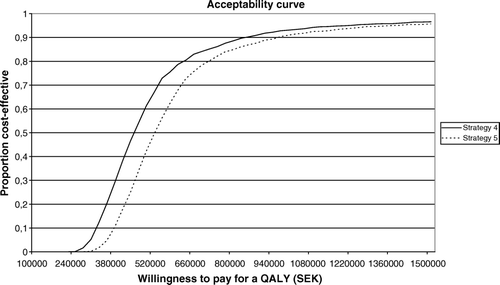
A probabilistic sensitivity analysis was performed in order to test the stability of the base case scenario using the distributions of costs and utility. The result of the probabilistic sensitivity analysis is presented graphically in .
Discussion
In the Swedish national guidelines for breast cancer treatment Citation[27], IHC testing is recommended for all metastatic breast cancer patients, with FISH confirmation of 2+ and 3+, and trastuzumab in combination with chemotherapy is recommended for patients with HER2 overexpression/gene amplification.
For an intervention to be considered cost-effective, the cost per QALY gained has to be at or below a specific cost-effectiveness threshold. There is currently no general consensus on the exact cost per QALY gained that defines this cost-effectiveness threshold. Several different methods have been proposed to estimate the value of a QALY. However, these methods produce drastically different results, with the estimated value of a QALY ranging from approximately 190 000 SEK to 3 200 000 SEK Citation[28]. The study by Persson et al. Citation[29] estimated the value of a QALY in a Swedish setting to 655 000 SEK, by using statistics on fatal road accident, life-expectancy and the value of a statistical life applied by the Swedish Road Administration when performing cost-benefit analysis in road safety investments.
In our analysis, we estimated the cost per QALY gained to be about 485 000 SEK for the strategy of IHC testing for all patients, with FISH confirmation of 2+ and 3+, and trastuzumab and chemotherapy treatment for FISH positive patients (Strategy 4). The cost per QALY gained was estimated to be about 561 000 SEK for the strategy of FISH testing for all patients, with trastuzumab and chemotherapy for FISH positive patients (Strategy 5). Using 655 000 SEK as the cost-effectiveness threshold, these strategies are considered cost-effective.
The study by Elkin et al. Citation[13] estimated the cost per QALY gained to $ 125 000 (approximately 992 000 SEK) for initial HercepTest for all patients, confirming 2+ and 3+ with FISH, followed by trastuzumab and chemotherapy for positive patients. The cost per QALY gained was estimated to 145 000 (approximately 1 151 000 SEK) for FISH testing for all patients, followed by trastuzumab and chemotherapy for positive patients. There can be several explanations to these relatively high estimates compared to our own. Since we found the risk of breast cancer related death to have substantial impact on the cost per QALY gained, the fact that we used clinical data from a different randomised study than Elkin et al. Citation[13] could explain some of the discrepancy. The risk of progression and breast cancer related death used in our model were based on the trastuzumab + docetaxel arm and the docetaxel alone arm (no cross over) from the Marty et al. study Citation[7]. Elkin et al. Citation[13] based their risks on the study by Slamon et al. Citation[6], where 66% of the patients in the chemotherapy alone arm crossed over and received trastuzumab after disease progression. Since it is chemotherapy alone, and not chemotherapy alone with possible crossover to trastuzumab that is being evaluated, using the OS for patients that have crossed over would tend to underestimate the real difference in OS between the two treatment arms that are evaluated in the model, and thus overestimate the cost per QALY gained. Though the authors implemented adjustments for this problem, it is difficult to judge the magnitude these adjustments had on the cost per QALY gained. Another reason for our lower estimated cost per QALY gained could be the utility scores used in the analysis. In the state progressive disease Elkin et al. Citation[13] used a utility score of 0.49 compared to our 0.685. Since we found that a decrease in the utility score for patients with progressive metastatic disease increased the cost per QALY gained, this difference in utility score is likely to impose a difference in the resulting cost per QALY gained between our study and the study by Elkin et al. Citation[13].
The study by Norum et al. Citation[14] estimated that cost per life year (LY) gained by trastuzumab and chemotherapy ranged between € 63 000 SEK and € 162 000 SEK (approximately 595 000 SEK and 1 531 000 SEK). These values are substantially higher than our estimates for the cost per LY gained, and the authors did not consider the treatment cost-effective. The higher values reported by Norum et al. compared to our results are due to the survival figures used in their analysis. They compare trastuzumab + chemotherapy with chemotherapy alone (cross over to trastuzumab) instead of comparing trastuzumab + chemotherapy with chemotherapy alone (no cross over), which causes them to overestimate the cost per LY gained.
In the study by Hornberger et al. Citation[11], the cost per QALY gained for patients with 3+ overexpression treated with trastuzumab and chemotherapy was estimated to £ 37 500 (approximately 540 000 SEK). In this study, the authors adjusted for the cross-over effect in the chemotherapy alone arm. The estimated cost per QALY gained was considered cost-effective by the authors, and is consistent with our estimates.
In our sensitivity analysis, we found that utility scores used had an important influence on the cost per QALY gained. Some argue that social tariffs or community–based samples are the more appropriate choice for economic evaluations to be used for policy decision, since they more closely reflects societal preferences Citation[30]. The utility scores of 0.685 used in the base case scenario, based on EQ-5D responses from Swedish breast cancer patients, represent such a value Citation[26]. However, others argue that only patients are able to accurately provide a reliable indicator of the subjective trade-off between quality and quantity of life of an individual Citation[31]. The time trade-off (TTO) is a method to elicit utility scores directly from patients. Using the utility scores based on the mean TTO values of 0.82 from the study by Lidgren et al. Citation[26] for metastatic breast cancer patients, Strategy 4 had a cost per QALY gained of 405 000 SEK, and Strategy 5 had a cost per QALY gained of 469 000 SEK.
As could be expected, the risk of breast cancer related death also had an important influence on the cost per QALY gained. Changes in this parameter translated into changes in survival predicted by the model. This highlights the importance of using OS data from patients that have not crossed over to trastuzumab after progression, in order to avoid an underestimation in the OS difference between patients receiving trastuzumab and chemotherapy compared with patients receiving only chemotherapy, which would tend to overestimate the cost per QALY gained for patients receiving trastuzumab. However, it should be noted that if the patients that cross over are different compared to the patients that do not cross over, this would bias the results of our base case scenario. If patients crossing over are healthier (sicker) compared to the patients not crossing over, then the base case scenario would tend to overestimate (underestimate) the difference in OS between the groups, which in turn would lead to an underestimation (overestimation) of the cost per QALY gained.
The transition probabilities used in our base case scenario translates into an OS of 31.2 months for patients receiving trastuzumab + docetaxel and 16.6 months for patients receiving docetaxel alone (no cross over), which is consistent with the results from the study by Marty et al7. If we instead use transition probabilities that translates into an OS of 22.1 months for patients receiving trastuzumab + docetaxel and 18.4 months for patients receiving docetaxel alone (with 66% cross over), which is consistent with the study by Slamon et al. Citation[6], the cost per QALY gained from Strategy 4 would be 1 123 000 SEK and the cost per QALY gained from Strategy 5 would be 1 388 000 SEK. However, since these figures include a 66% cross over, the estimated cost per QALY gained is substantially overestimated.
The sensitivity and specificity of the IHC test also had an impact on the cost per QALY gained. Increases in the sensitivity and specificity had a small impact on the cost per QALY gained for Strategy 4, but a substantial impact on the cost per QALY gained for Strategy 5. This is expected since an increase in the sensitivity and specificity of the IHC test, will decrease the additional benefit offered by FISH testing, thus increasing the cost per QALY gained for Strategy 5.
In the base case scenario, we assumed that in the absence of HER2 gene amplification, the patient received no additional benefit from trastuzumab therapy Citation[17–19]. In the study by Marty et al. Citation[7], 88% of the study population were 3+ by IHC test, 12% were FISH positive, and 97% were 3+ and/or FISH positive. Instead assuming that benefit from trastuzumab treatment is only derived if patients have 3+ by IHC test, we can compare the strategy of using IHC testing for all patients with trastuzumab and docetaxel for 3+ patients (Strategy 2), with the strategy of docetaxel alone for all patients (Strategy 1). In this analysis we estimated the cost per QALY gained for Strategy 2 to 474 000 SEK, which is considered cost-effective when using a cost-effectiveness threshold of 655 000 SEK. This result is consistent with our results from the analysis where we assumed that only patients with HER2 gene amplification received benefit from trastuzumab therapy. This corroborates our findings that trastuzumab and docetaxel seems to be a cost-effective treatment option for patients with HER2 overexpression/gene amplification.
It has been argued that the difference between consumption and production during life years gained should be included as a cost (usually referred to as future cost) in cost-effectiveness analysis Citation[32], Citation[33]. Estimates for the difference between consumption and production in different age groups for the Swedish general population have been used previously in cost-effectiveness analysis Citation[34]. However we have no data on the difference between consumption and production for metastatic breast cancer patients. Using the annual difference between consumption and production for individuals in the general population individuals aged 65–74 in the study by Ekman et al. Citation[34], the cost per QALY gained for Strategy 4 was estimated to 692 000 SEK, and the cost per QALY gained was estimated to 769 000 SEK for Strategy 5. But since these estimates are based on the difference between consumption and production for the general population and not metastatic breast cancer patients, these estimates should be interpreted with caution.
It should be noted that the cost-effectiveness analysis is a tool for efficiency consideration and not equity considerations Citation[35]. Also, cost-effectiveness analysis doesn't take into consideration the budget impact of using a certain drug. Even if trastuzumab treatment is cost-effective, if the high cost per patient treated with trastuzumab prevents some physicians in certain clinics to give patients the best available treatment due to budget constraints, problems with equity can arise. Patients that are treated at clinics that can afford trastuzumab treatment could potentially receive better care compared to patients that are treated at clinics that cannot afford trastuzumab treatment.
It is also important to note that our study have several limitations. One limitation of our study is that the data on time to progression and overall survival is based on data from the clinical study by Marty et al. and the patient cohorts included in this clinical trial might not be overall representative for an average Swedish metastatic breast cancer patient. However, the impact this could have on the clinical outcome is difficult to quantify.
Also, time to progression and overall survival in the model was calibrated to median values given in the study by Marty et al. More detailed clinical data would make it possible to more accurately model the time to progression and overall survival. This highlights the importance of successive economic evaluations of trastuzumab and chemotherapy in the metastatic setting as more detailed data becomes available.
Another limitation of the analysis is the validity of the cost and utility data, which were collected from patients in the Stockholm County, and hence might not be directly generalizable to other countries or other regions in Sweden.
Conclusion
Our analysis indicate that the Swedish guidelines of IHC testing for all patients, with FISH confirmation of 2+ and 3+, and trastuzumab and chemotherapy treatment for FISH positive patients is a cost-effective treatment option compared to chemotherapy alone for all patients. However, according to our analysis, this strategy is only preferable to the strategy of FISH testing for all patients with trastuzumab and chemotherapy treatment for FISH positive patients if our willingness to pay for a QALY is in the range of 485 000 SEK to 561 000 SEK. FISH testing for all patients with trastuzumab and chemotherapy treatment for FISH positive patients is the treatment strategy associated with the longest quality adjusted survival in our analysis, as well as having an incremental cost-effectiveness ratio lower than commonly used willingness to pay thresholds. This indicates that FISH testing for all patients with trastuzumab and chemotherapy treatment for FISH positive patients is cost-effective, and the preferable treatment strategy from a societal perspective.
References
- The National Board of Health and Welfare. Cancer Incidence in Sweden 2004. Stockholm: Official Statistics of Sweden; 2005.
- The National Board of Health and Welfare. Causes of death 2003. Stockholm: Official Statistics of Sweden; 2005.
- Lidgren M, Wilking N, Jönsson B. Cost of breast cancer in Sweden 2002. Eur J Health Econ 2007; 8: 5–15
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235(4785)177–82
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244(4905)707–12
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92
- Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol 2005; 23: 4265–74
- Svenska bröstcancergruppen. Nationellt vårdprogram för bröstcancer. Stockholm; 2003.
- Apoteket AB. Apotekens totala försäljning av humanläkemedel 2003–2005. http://www.apoteket.se. Accessed 2006-08-05.
- Läkemedelsindustriföreningen. Swedish pharmaceutical reference book. www.fass.se. Accessed August 30th, 2006.
- Hornberger J, Kerrigan M, Foutel V. Cost-effectiveness of trastuzumab (herceptin) for treatment of metastatic breast cancer. ESMO Poster 2002.
- The National Institute for Clinical Excellence. Guidance on the use of trastuzumab for the treatment of advanced breast cancer - Technology Appraisal Guidance-No.34. London: The National Institute for Clinical Excellence; 2002.
- Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. HER-2 testing and trastuzumab therapy for metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol 2004; 22: 854–63
- Norum J, Risberg T, Olsen JA. A monoclonal antibody against HER-2 (trastuzumab) for metastatic breast cancer: A model-based cost-effectiveness analysis. Ann Oncol 2005; 16: 909–14
- Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 2000; 18: 3651–64
- Press MF, Slamon DJ, Flom KJ, Park J, Zhou JY, Bernstein L. Evaluation of HER-2/neu gene amplification and overexpression: Comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol 2002; 20: 3095–105
- Mass R, Press M, Andersson S, Murphy M, Slamon D. Improved survival benefit from Herceptin (trastuzumab) in patients selected by fluorescence in situ hybridization (FISH). Proceedings of ASCO. 2001;20:( Abstract 85).
- Vogel CL, Cobleigh M, Tripathy D, Mass R, Murphy M, Stewart SJ. Superior outcome with herceptin (trastuzumab) (H) in Fluorescence in Situ Hybridization (FISH)-Selected Patients. Proceedings of ASCO 2001;20:( Abstract 86).
- Press M, Slamon D, Cobleigh M, et al. Improved clinical outcomes for Herceptin treated patients selected by fluorescence in situ hybridization (FISH). Lab Invest 2002:( Abstract 185).
- Statistics Sweden. 100 - Life tables. Statistical Yearbook of Sweden 2006. Stockholm: Statistics Sweden; 2005.
- Statistics Sweden. Vikt och längd i befolkningen. http://www.scb.se/templates/tableOrChart_47966.asp. Accessed August 30th, 2006.
- Lidgren M, Wilking N, Jönsson B, Rehnberg C. Resources use and costs associated with different states of breast cancer. Int J Technol Assess Health Care 2007; 23: 223–31
- The National Board of Health and Welfare. Vårdkostnader 2002 för NordDRG. http://www.socialstyrelsen.se/NR/rdonlyres/125A3FCE-B0AA-4B9B-ADBF-83533F01CEA2/2857/200412511.pdf. Accessed October 24th, 2006.
- Statistics Sweden. 407 - Consumer price index. Statistical Yearbook of Sweden 2006. Stockholm: Statistics Sweden; 2005.
- Södra regionsvårdsnämden. Universitetssjukhusen Lund och Malmö. http://www.srvn.org/pris06/26_92.pdf. Accessed October 7th, 2006.
- Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res 2007.
- Svenska bröstcancergruppen. Nationellt vårdprogram för bröstcancer. v 1.5:, http://www.swebcg.roc.se/natriklinjbrca.htm. Accessed October 6th, 2006.
- Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: In search of a standard. Med Decis Making 2000; 20: 332–42
- Persson U, Hjelmgren J. Hälso- och sjukvården behöver kunskap om hur befolkningen värderar hälsan. Läkartidningen 2003; 100: 3436–7
- Gold M, Siegel J, Russel L, Wienstein M. Cost-effectiveness in health and medicine. Oxford University Press, New York 1996
- Johannesson M. Theory and methods of economic evaluation of health care. Kluwer Academic Publisher. 1996
- Meltzer D. Accounting for future costs in medical cost-effectiveness analysis. J Health Econ 1997; 16: 33–64
- Johannesson M, Meltzer D, O'Conor RM. Incorporating future costs in medical cost-effectiveness analysis: Implications for the cost-effectiveness of the treatment of hypertension. Med Decis Making 1997; 17: 382–9
- Ekman M, Zethraeus N, Dalström U, Höglund C. Kostnadseffektivt att behandla kronisk hjärtsvikt med bisoprolol. Läkartidningen 2002; 99: 646–50
- Karlsson G, Johannesson M. The decision rules of cost-effectiveness analysis. Pharmacoeconomics. 1996; 9: 113–20