Abstract
During the time period 1977–2007 postoperative radiotherapy in DBCG has varied considerably with regard to techniques and indications together with changes in the extent of surgery and adjuvant systemic therapy. The radiation treatment has been developed on the basis of clinical, radiophysical and radiobiological principles, encompassing also practical problems such as available equipment in the different centres and at times lack of sufficient machine capacity.
The paper focus especially on the comprehensive work done prior to the DBCG 82 b&c studies, in order to optimize radiotherapy in all aspects prior to the evaluation of the efficacy of this treatment modality. The results from these trials did succeed in clear evidence that radiotherapy has an important role in the multidisciplinary treatment of early breast cancer. In parallel to these studies a new and challenging use of radiotherapy after breast conserving surgery was evaluated in the DBCG TM 82 protocol. The experience obtained with different techniques in this study formed the basis for the current principles of radiotherapy after lumpectomy.
Reduction of radiation related morbidity has been a major issue for the DBCG radiotherapy group, and in this aspect several studies, including quality control visits, have been carried out to make the relevant modifications and to evaluate deviations from the guidelines between the centres. The background for the changes in radiotherapy is described for each of the programme periods as well as future perspectives which will include further refinements of the target and adjustments of dose and fractionation in selected patients.
In the beginning of the 1970's the standard treatment for early breast cancer in Denmark was simple mastectomy followed by loco-regional radiotherapy according to the results from the Copenhagen study comparing simple mastectomy plus radiotherapy versus radical mastectomy alone and similar studies Citation[1–4]. No adjuvant systemic therapy was recommended. Most patients were treated with orthovoltage equipment with the McWhirter technique where the dose aim was 40–45 Gy in 18–20 fractions over 3 weeks. However, during this time period more and more megavoltage equipment was installed in the larger radiotherapy departments and therefore treatment techniques for postoperative radiotherapy in early breast cancer with megavoltage irradiation were developed. Typically the treatment technique included an anterior field against the lymph nodes in the periclavicular and axillary region in combination with tangential opposed fields to the chest wall. The position of the fields were planned and verified on a simulator by the use of bone structures as landmarks. The dose aim was 54 Gy in 24 fractions maximum dose in the anterior photon field whereas the dose for the tangential fields was calculated at the central axis between the two fields corresponding to the later ICRU-reference point. Dose homogeneity was aimed for by the use of standard wedges. The extension of the photon fields was individualized according to the location of the primary tumour and the loco-regional spread. At that time the target definitions which nowadays form the basis of dose planning were just under development and the first ICRU report including target definitions was published in 1978 (ICRU 29) Citation[5].
Parallel to the development in radiotherapy with more effective high-energy equipment and new treatment techniques the first convincing results of adjuvant systemic therapy in early breast cancer Citation[6], Citation[7] appeared in the mid seventies. Especially pre-menopausal women with node positive disease seemed to obtain a pronounced improvement in disease-free survival. These results led to a paradigm shift in the treatment of early breast cancer towards a more multidisciplinary approach, and it was within this time period that the DBCG group was formed, and encouraged also to evaluate adjuvant systemic therapy together with optimal loco-regional therapy in high-risk patients Citation[8], Citation[9].
In the following the important and major changes in the radiotherapy recommendations during 30 years in DBCG are described in relation to the different programmes.
Radiotherapy in DBCG 77
One of the major aims of the DBCG organization Citation[8] was to develop national treatment strategies to assure a uniform treatment throughout the country and to allow conduction of national clinical trials. With the introduction of the DBCG-77 protocols it was decided that total mastectomy and axillary sampling should be offered to all patients with early breast cancer. By this pathologic staging the patients could be divided into a low-risk group (node negative and T ≤5 cm) or a high-risk group (node positive and/or invasion to the pectoral fascia and/or T3 or T4 tumours). Patients belonging to the low-risk group were not recommended further treatment neither postoperative radiotherapy nor adjuvant systemic therapy. High-risk patients were treated with postoperative loco-regional radiotherapy as a standard or included in DBCG protocol-77b or DBCG-77c Citation[10–12]. In the 77b study the value of different adjuvant systemic chemotherapy or immunotherapy was studied in addition to postoperative radiotherapy. In the protocol DBCG 77c endocrine therapy or immunotherapy in addition to postoperative radiotherapy was evaluated in all postmenopausal women without any upper age limit. The protocols included recommendations for the postoperative radiotherapy (). Due to the protocol design which described simultaneous chemotherapy, it was decided to modify the total dose aim to avoid serious acute skin reaction due to interaction with radiation (). Publications and clinical experience had shown enhanced normal tissue reactions (acute and late) in patients who received simultaneous chemotherapy and radiotherapy Citation[13]. For practical reasons it was decided to keep the same dose aim in all treatment groups irrespective of type of systemic therapy or none. As mentioned above the description of target definitions and target depth doses was about to be described in the ICRU 29 Report. This was known by the oncologists and physicists in the DBCG group and included as a recommendation for the dose calculation. Priority was made to obtain the same minimum dose at depth in the axilla to assure optimal tumour control in all patients irrespective of thickness of the patient. The reference point for the dose calculation in the axilla was chosen to be the midaxilla (MA) corresponding to the half of the anterior-posterior (a-p) diameter in cm at the bottom of caput humeri (). This magic reference point showed to be relevant also for the definition of the axillary target depth in later DBCG programmes.
Figure 1. The x-ray used for the target determination showing the reference point for the MA measurements. The levels of the CT-slices are indicated at the margin. The position of the patient is not standard because of the small scan diameter of the CT-scanner.
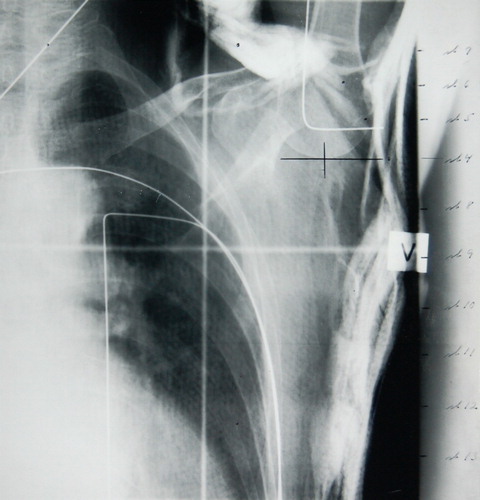
Table I. DBCG Recommendations for postmastectomy radiotherapy 1977–2007.
To reach the minimum dose according to increasing depths of the midaxilla in different patients it was necessary to increase maximum doses as shown in . However, the upper limit was set to be 54 Gy. There were two treatment techniques available. A new three-field technique was introduced using an anterior photon field against the periclaviculary and axillary node areas combined with one anterior electron field covering the remaining part of the chest wall (). It was not intended to include the internal mammary nodes. The other available technique was an anterior photon field to the periclavicular and axillary regions combined with two tangential opposed photon fields to the chest wall.
Figure 2. Left: The upper curve shows the common peak dose of 54.0 Gy in 24 fractions for all patients and the lower curve indicate the dose to the later defined the midaxilla (MA) target depth. Middle: The lower curve is the prescribed minimum dose to the target and the upper curve is the corresponding peak dose. For big a-p diameters the minimum dose drops down due to the upper dose limit of 54.0 Gy for the peak dose.Right: The median dose concept results in symmetrical peak and minimum doses. The demand for a dose deviation less than + 10% cause a energy shift for big a-p diameters.
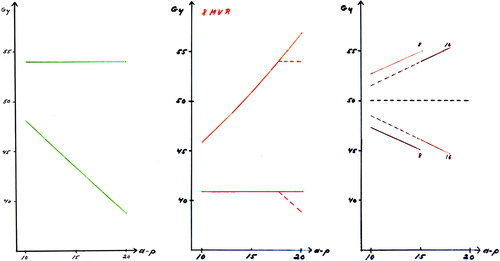
Figure 3. DBCG 77: I: Anterior photon field including supra/infraclavicular region arid axillary region, lI: Bolus covered part of the anterior photon field. III: Anterior electron field. A: Reference point for mid-plane of axillary field and measurement of anterior-posterior diameter (A-P diameter). For further details, see Overgaard et al. Citation[18]. DBCG 82. Left: The anterior combined photon/electron 3-field technique which was most commonly used in the DBCG 82 b&c trials. Field I: anterior photon field. Fields II and III: anterior electron fields. Larynx and shoulder joint are blocked and a wax bolus covers the scar area in the photon field area. Right: The wide tangential field technique. Field I: anterior photon field, with blocking of larynx and shoulder joint. Fields II and II: opposed tangential photon fields with the medial field border being located 3 cm contralateral to midsternum. From Nielsen et al. Citation[45]. DBCG 89. Treatment fields to patients with ≥ 10 nodes removed. Left figure: Anterior photons fields to periclavicular and level 3 axillary nodes and lateral chest wall with lung blocks. Right figure: Anterior electron field covering remaining part of chest wall and IMN.DBCG 03. Treatment fields to patients with ≥ 10 nodes removed. Anterior combined photon and electron fields covering periclaviculary area, level 3 axillary nodes and chest wall. IMN only included in patients with right-sided tumours.
![Figure 3. DBCG 77: I: Anterior photon field including supra/infraclavicular region arid axillary region, lI: Bolus covered part of the anterior photon field. III: Anterior electron field. A: Reference point for mid-plane of axillary field and measurement of anterior-posterior diameter (A-P diameter). For further details, see Overgaard et al. Citation[18]. DBCG 82. Left: The anterior combined photon/electron 3-field technique which was most commonly used in the DBCG 82 b&c trials. Field I: anterior photon field. Fields II and III: anterior electron fields. Larynx and shoulder joint are blocked and a wax bolus covers the scar area in the photon field area. Right: The wide tangential field technique. Field I: anterior photon field, with blocking of larynx and shoulder joint. Fields II and II: opposed tangential photon fields with the medial field border being located 3 cm contralateral to midsternum. From Nielsen et al. Citation[45]. DBCG 89. Treatment fields to patients with ≥ 10 nodes removed. Left figure: Anterior photons fields to periclavicular and level 3 axillary nodes and lateral chest wall with lung blocks. Right figure: Anterior electron field covering remaining part of chest wall and IMN.DBCG 03. Treatment fields to patients with ≥ 10 nodes removed. Anterior combined photon and electron fields covering periclaviculary area, level 3 axillary nodes and chest wall. IMN only included in patients with right-sided tumours.](/cms/asset/5ecdf8a5-d940-49f9-9b76-7fa1f619dbe6/ionc_a_307974_f0003_b.jpg)
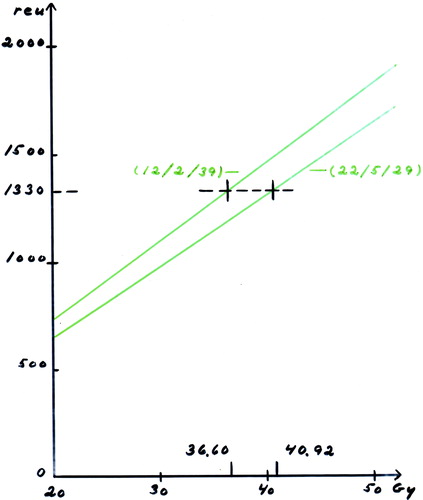
Due to the fact that there was no access to megavoltage treatment for patients living in peripheral parts of the country it was allowed to offer these patients orthovoltage treatment ad modem McWhirter Citation[14]. Moreover, even in the larger departments the machine capacity was not large enough to accumulate all patients referred for radiotherapy. Therefore, in four of the centres it was decided to use hypofractionation with 2 weekly fractionations allowing the double number of patients to be treated. The fifth megavoltage centre continued to give conventional 5 fractions per week. The two fractionation schedules were thus a minimum of 36.6 Gy in 12 fractions with 2 fractions per week or 40.92 Gy in 22 fractions with 5 fractions per week, corresponding to a CRE value of 1335 reu. The Ellis NSD formalism and the CRE- derivative by Kirk et al were used to calculate isoeffects between these two regimens , Citation[15], Citation[16].
Figure 4. Determination of the two equivalent doses (no of fx/fx per wks/total days) used for the recommended fractionations.
The loco-regional radiotherapy in the DBCG 77 protocols resulted in satisfactory loco-regional tumour control especially in patients, who were also treated with systemic therapy Citation[12]. However, in the departments using the hypofractionation schedules it became obvious after two years that the radiation morbidity had increased remarkably compared to previous experience. Impairment of shoulder movements, lymph oedema, severe fibrosis in the irradiated areas, spontaneous rib fractures and lung fibrosis occurred much more frequent than expected Citation[17], Citation[18]. Therefore in one of the centres, Aarhus, it was decided before the end of the protocol period to stop the use of hypofractionation and switch to conventional fractionation. This later allowed comparison between the early and late normal tissue effects in patients treated with these two schedules with and without adjuvant systemic therapy Citation[17–30], Furthermore has the detailed morbidity recording in these patients together with access to genetic material and fibroblast cell cultures from the patients given us the basis for research and identification of genetic profiles for risk of radiation induced morbidity Citation[31–42].
Radiotherapy in DBCG 82
In contrary to the increased radiation morbidity in the DBCG 77 programmes the preliminary results of the effect of adjuvant systemic therapy appeared in the beginning of the 80's showing significant improvement in disease-free survival Citation[10–12]. These results supported the Fisher theory Citation[43] which says that breast cancer is mainly a systemic disease and therefore only improvements can be obtained with more effective systemic therapy whereas more aggressive loco-regional therapy with more extensive surgery and/or radiotherapy was not likely to improve overall results as stated in the Halsted theory Citation[44]. Thus, in the light of the recent bad experience with unacceptable radiation morbidity together with the general opinion which held to the theory, that postoperative radiotherapy would not improve overall survival, it was decided to design a trial to evaluate whether postoperative radiotherapy is necessary in high-risk breast cancer patients who also receive adjuvant systemic therapy. The 82b and c protocols were designed as two three-arm studies comparing postoperative radiotherapy after mastectomy and systemic therapy versus the same systemic therapy alone versus the same systemic therapy plus additional systemic therapy in high-risk patients Citation[10–12].
Due to the experience with increased radiation morbidity in the previous protocol, the guidelines for radiotherapy in the DBCG 82 protocols were comprehensively prepared to optimize the radiation treatment in all respects. It was decided to follow the ICRU target definitions. To obtain consensus about the exact target volume to include, the members of the radiotherapy committee were asked to draw target volumes on a number of CT slices of a patient in treatment position as shown in and . Further, it was decided that the target volume should include all loco-regional node areas (periclavicular, axillary, and internal mammary nodes (IMN)) as well as the chest wall with sufficient margin around the surgical scar. After consensus about the target definitions it was possible to make field arrangements on a simulator to cover these areas as seen in . An anterior photon field could cover the deep part of the target in the axilla and the periclaviculary area, whereas the more superficial part of the target on the chest wall and IMN area could be covered by anterior electron fields. Following this procedure in one patient, a number of patients of different sizes were scanned to test the variation of the target between patients. On the basis of these information the DBCG 82b&c irradiation techniques was developed and described and thus it was possible to make field arrangements in every patient using only a simulator. In it is demonstrated how the anterior photon field was angled to improve the junction between the photon and electron field. The choice of electron energy for the IMN field and the chest wall was based on ultrasound measurements of the distance between skin surface and pleural surface at relevant points in both fields. Although most patients were treated with the anterior three-field technique using a combination of photon and electron fields the, guidelines also included recommendations for a three-field photon technique using wide tangents to include the chest wall and internal mammary nodes combined with an anterior periclavicular field (Figure 3).
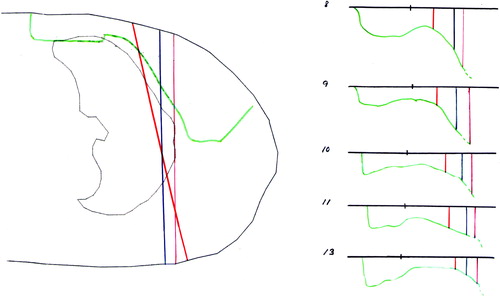
Figure 5. Some of the proposals for the target. The green line indicates the DBCG target. The slice shows the situation at the midaxilla (MA) reference point.
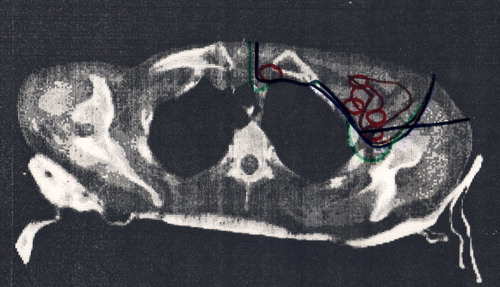
Figure 6. The depth of DBCG target defined from CT slices with 2 cm interval. The depth of the dashed curves is equal to the corresponding colored curves plus 0.5 cm.

Figure 7. A scan in the thorax region with indication of the lung and the DBCG target. The tree colored lines point out the borderlines between the photon and electron field. The magenta line marks a vertical beam at the inside of the thorax wall. The blue line is situated 1 cm inside the lung. The red line shows the situation with the gantry turned 10-15 degrees from vertical position and the borderline 1 cm inside the lung defined by an x-ray. At the right side of the figure the depth of the DBCG target is indicated by the green lines for a number of slices. The tree colored lines marks the borderlines between the photon and electron field. The black dot at the surface shows the separation of the parasternal and the scar region.
To avoid interaction with chemotherapy it was chosen to give sequential radiotherapy and chemotherapy with a one to two weeks interval between start and end of radiotherapy and chemotherapy ( and ). Further standard fractionation schedules were chosen, either 50 Gy in 25 fractions over 5 weeks or 48 Gy in 22 fractions in 4 fractions per week Citation[12], Citation[45]. In addition, it was planned to record all individual measurements and doses as well as a prospective registration of acute and late radiation morbidity Citation[45], Citation[46].
The requirements about minimum dose at the deep part of the target in the axilla were replaced by the use of median dose to the target, which includes a dose variation between +/− 10% for the photon fields as seen in the right panel in . The calculation of the dose to the individual fields was based on standard tables and therefore dose contributions from transmission or scatter from the other fields were not included (). At a later revision of the dose in the 89 programme, the knowledge of these dose contributions resulted in a reduction of the dose to 48 Gy in 24 fractions to compensate for the more intensified chemotherapy.
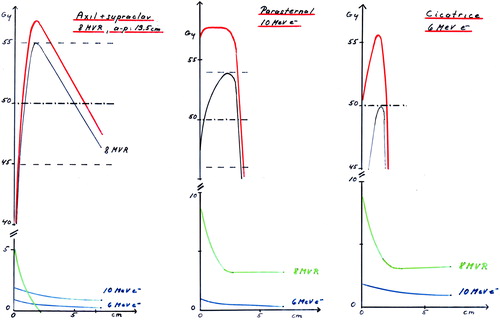
Figure 8. Left: The depth dose curve for the open field is shown in black. To this curve we have to add the dose from electron contamination from the Perspex tray (green) and the transmitted photon dose from the electron fields. Middle: To the standard 10 MeV electron depth dose we must add the contribution from the photon field containing electron contamination and photon transmission. And on top of that the photon transmission from the electron field adds up. Right: The same situation as in Figure 6b but the transmitted dose is higher due to the higher electron energy.
A comprehensive audit of the radiotherapy used in the 82b and c protocols as well as the outcome with respect to loco-regional and distant tumour control has been described in details elsewhere Citation[45–47]. Overall, the addition of post-mastectomy radiotherapy in high risk pre- and post-menopausal patients in spite of adjuvant systemic therapy has demonstrated a substantial benefit in terms of loco-regional tumour control and improved long-term survival Citation[48–52]. Thus, these results have clearly demonstrated the proof of principle that primary loco-regional tumour control has impact on survival even in patients who also receive an adjuvant systemic therapy Citation[53]. Ongoing studies based on material from the tumour biobank in the DBCG 82b and c protocols are underway to identify genetic and molecular targets which in details may identify the patients who especially may benefit from postmastectomy irradiation Citation[54–57].
With regard to radiation morbidity, this has been evaluated in details by examination of all patients in one centre. The main conclusion is that the late morbidity caused by irradiation was mild and only moderately increased compared to non-irradiated patients. Further, the registry based study of all patients with respect to cardiac morbidity and mortality did not demonstrate radiation related cardiac morbidity within 12 years of observation Citation[58], Citation[59]. Neither have preliminary results of 25 years’ follow-up of the DBCG 82b and c protocols indicated increased cardiac mortality in irradiated patients.
Radiotherapy in DBCG 82 TM
Parallel to evaluation of post-mastectomy irradiation in the DBCG 82b and c protocols, the concept of breast conserving therapy was planned Citation[60], Citation[61] and evaluated in the TM 82 protocol Citation[62–65]. The majority of the patients included in this protocol were low risk (node negative and tumour ≤5 cm) and therefore the target in patients randomized to breast conserving therapy was only the residual breast including underlying chest wall, whereas similar patients treated with mastectomy did not receive postoperative radiotherapy. However, high-risk patients included in this protocol irrespective of primary surgery received loco-regional postoperative radiotherapy to target areas analogue to what is described in 82 b and c protocols. The guidelines for radiotherapy after breast conserving surgery and axillary dissection were described separately as given in . Two treatment techniques were described for treating the breast. Most patients were treated with tangential photon fields using supervoltage 6–10 MV photon beams to the breast. But in 3 centres an anterior electron field including all breast tissue was chosen, preferably in patients with small breasts. Wax compensation was used around the breast to even out the distance from the beam entrance to the pleural surface assessed by ultrasound. Electron energies from 6–20 MeV were used, and the prescribed dose was 50 Gy median absorbed dose with the 85% isodose line at the pleural surface Citation[66].
Table II. DBCG recommendations for radiotherapy after breast conserving surgery (BCS) 1977–2007.
At that time the skin 3 cm around the lumpectomy scar was included in the target and therefore bolus was used during treatment. However, if severe skin reaction occurred, bolus was discontinued during remaining treatment as well as during boost treatment. All patients received a boost of 10 to 24 Gy in 5 to 12 fractions. Most patients were treated with electron boost fields, but photon fields were used in patients with a deep seated tumour bed and interstitial brachytherapy was used in one department where this was technically possible. The cosmetic outcome in relation to the different treatment techniques, has been evaluated in details as described in Citation[66–68].
All patients surviving more than 3 years after initial treatment without recurrence in the group treated with lumpectomy and radiotherapy were called for examination with the purpose of evaluating the cosmetic outcome in relation to different treatment techniques. On the basis of this study Citation[66] the electron technique including whole breast irradiation was found to be inferior to the photon technique and therefore this treatment technique was abandoned from 1993. The evaluation of the cosmetic results in this study also lead to corrections of the surgical technique, especially by avoiding the same incision for axillary dissection and lumpectomy in laterally localized tumours. Finally, the use of bolus on the lumpectomy scar was also found to impair the cosmetic outcome and therefore the use of bolus was later stopped.
In the DBCG 82 TM protocol the intention was only to use conventional fractionation in patients receiving radiotherapy after breast conserving surgery.
Radiotherapy in DBCG 89
The DBCG 89 protocols mainly addressed questions regarding more specific and more intensive systemic therapy on the basis of predictive and prognostic factors, such as oestrogen receptor status and prognosis in relation to malignancy grade. Regarding the loco-regional treatment, only preliminary results from the DBCG 82b and c protocols were available Citation[48]. A very high incidence of loco-regional recurrence was found in both pre-and postmenopausal women randomized to systemic therapy alone compared to patients treated with postoperative radiotherapy plus the same systemic therapy. But in a small sub-group, namely patients younger than 45 years with 4 or more positive nodes, a significant decrease in survival was found when treated with systemic therapy alone Citation[12]. However, the interpretation of these early results by the DBCG Steering Group was, that the high rate of loco-regional recurrences could mainly be explained by the insufficient axillary dissection and the rather weak CMF schedule used in the DBCG 82b protocol and therefore might not solely indicate radiotherapy in all high risk patients in the 89 programmes. Along with that the international consensus about postmastectomy radiotherapy in the late 1980's was very negative since results from overview analyses had shown long term negative outcome of postoperative radiotherapy with excess mortality in irradiated patients who survived 10 or more years after primary treatment compared to similar non-irradiated patients Citation[69]. Therefore it was decided by initiation of the DBCG 89 protocols that postmastectomy irradiation was only indicated in patients, who had tumour invasion through the pectoral fascia and in patients younger than 45 years with 4 or more positive nodes. The treatment techniques for postmastectomy irradiation remained unchanged from the 82 period and the combined anterior photon/electron 3-field technique became the preferred technique (). However, in node negative patients who had invasion into the pectoral fascia, the target included only the chest wall.
Since all orthovoltage units closed in the late 1980's, only megavoltage irradiation was allowed from 1989 and onwards ( and ).
Based on the results from the DBCG 82 TM study and similar randomized trials, which all confirmed that breast conserving surgery plus radiotherapy gave similar results to mastectomy, it was decided that breast conserving therapy could be offered as a standard treatment to eligible patients Citation[70]. Postoperative radiotherapy was found indicated in all patients after lumpectomy, but patients younger than 45 years with 4 or more nodes positive should also receive regional lymph node irradiation in addition to irradiation of the residual breast tissue. The two treatment techniques, either tangential photon fields to the breast or an anterior electron field as described above, continued as standard techniques [].
The dose to the loco-regional target irrespective of type of surgery was reduced to 48 Gy in 24 fractions with 5 fractions per week due to the timing of concomitant cyclophosphamide and radiotherapy and more intensive chemotherapy with 3 weeks’ interval and randomization to CEF vs. CMF in the DBCG 89d protocol [ and ] Citation[9]. Also the boost dose was reduced to 10 Gy in 5 fractions in patients having lumpectomy with a resection margin larger than 5 mm from the invasive tumour and to 16 Gy in 8 fractions in patients with a smaller margin or DCIS close to margin. The boost techniques remained unchanged from the previous protocol but it became mandatory for the surgeons to mark the tumour bed with clips and also to separate the incision for axillary dissection from the incision for lumpectomy in laterally localized tumour with the aim to reduce the boost volume ().
Table III. Timing of radiotherapy (RT) and chemotherapy in DBCG.
When the results from the follow-up examination of all patients in the DBCG 82 TM protocol became available in 1992, the anterior electron beam technique was abandoned due to inferior cosmetic results with marked late skin reactions such as teleangiectasia and dyspigmentation Citation[66], Citation[68]. At the same time the 4 fraction-per-week schedule, which had been used in some departments to be able to do machine checks during normal working hours, was stopped in addition to other minor corrections ().
Due to the very high recurrence rates in the DBCG 82 b and c studies, which in particular occurred in the axilla and on the chest wall, the surgeons did major efforts to improve the surgical technique, especially to improve the axillary dissection Citation[8], Citation[9]. Therefore from 1994 the requirements for a proper axillary dissection became the removal of at least 10 nodes compared to 4 nodes previously.
In the beginning of 1995 an analysis of the 82b study showed significant survival benefit in all pre-menopausal patients who after mastectomy received radiotherapy in addition to CMF. Therefore, the indications for postmastectomy irradiation and regional nodal irradiation after breast conservative surgery were extended to include all pre-menopausal node positive patients. To avoid complications such as lymph oedema and impairment of shoulder movements with a combination of radiation and more aggressive axillary surgery, the target in the axilla was reduced to include only level 3, whereas the periclaviculary and internal mammary nodes were included as previously described ( and ).
During the 1990's the radiotherapy guidelines were revised several times and quality control visits in all 6 Danish megavoltage centres were carried out in 1996. This process did later also include the Department of Radiotherapy, St. Franziskus-Hospital in Flensburg, which from 2000 became the seventh megavolt centre in DBCG. Since then this department has in close collaboration with the DBCG radiotherapy group treated patients from the southern part of Jutland following the DBCG guidelines and reporting the radiotherapy data to the central DBCG registry (Table I).
In 1999 the indications for post-mastectomy radiotherapy were extended to include also all post-menopausal patients due to publication of the results from the DBCG 82c protocol, which showed significant benefit of radiotherapy both in terms of local control and 10-year-survival, similar to the 82b study. Thus, during 1999 the number of patients, who should routinely receive postoperative radiotherapy increased dramatically and this lead to longer and longer waiting times and a need for larger treatment capacity in the country.
Radiotherapy in DBCG 99
In general, the indications for post-operative radiotherapy after mastectomy and lumpectomy have not changed since 1999. There is no upper age limit for standard radiotherapy after lumpectomy, whereas post-mastectomy irradiation in high-risk patients older than 70 years is based on individual evaluation of benefits versus risks in relation to medical conditions.
The timing of radiotherapy and chemotherapy has changed over the years as previously mentioned and described in . However, since 2001 it has been standard to give chemotherapy first, followed immediately by radiotherapy except in patients with residual tumour after surgery. There is no clear evidence neither from the literature nor from previous DBCG studies what the optimal timing is Citation[71–73].
Along with the installation of new equipments with CT dose planning systems and new treatment facilities including multi-leaf collimators it has been necessary to change the guidelines and revise the targets accordingly. Thus, it became clear that inclusion of the internal mammary nodes was not always sufficient with the previous standard techniques Citation[74]. Moreover it became clear that especially the use of wide tangential fields in patients who should receive loco-regional irradiation after lumpectomy, would often include large volumes of ipsilateraly lung, and also the anterior part of the heart, and sometimes also parts of the opposite breast. Thus a serious problem was defined, since there is clear evidence that inclusion of just a smaller volume of the anterior part of the heart will result in excess cardiac morbidity and mortality due to ischemic cardiac disease, which could counterbalance a moderate benefit from radiotherapy. Further, the standard adjuvant chemotherapy in most patients now includes more cardiotoxic drugs, such as anthracyclins and Herceptin. Analysis of a study including different doses of anthracyclins in combination with postoperative radiotherapy, including different volumes of the heart in the target has shown significant increase of cardiotoxicity Citation[75].
At present neither the previous DBCG protocols nor the international literature has proved the benefit of inclusion of IMN in high risk- patients. Only one large trial (EORTC 22922/10925) has addressed this question (closed in 2002 after allocation of more than 4 000 patients). Results from this trial will not be available until 5–10 years after stop of accrual. On the basis of these considerations it was decided that in high risk patients, irrespective of lumpectomy or mastectomy, the target should not include the internal mammary nodes in patients with left-sided breast cancer whereas the target in patients with right-sided breast cancer if possible should include the internal mammary nodes (). Following activation of these guidelines implemented in 2003, prospective registration of all radiotherapy data would allow a later analysis of the outcome in left-sided versus right-sided high risk patients with regard to tumour control, survival and cardiac morbidity. Currently techniques, which safely can assure minimal radiation dose to the lung and heart are under evaluation for routine use Citation[76], as well as the large RACE study which is aimed to define the risk targets Citation[77].
Since the beginning of 2000, registration of all radiotherapy data has been mandatory in all patients receiving radiotherapy, including information to the central registry why radiotherapy is not given if indicated ( and ).
Radiotherapy in DBCG 2007
With the development of CT based treatment planning in all patients Citation[78] much more detailed information about the coverage the target, dose homogeneity and of risk organs is available if electronically recorded. It is the ambition to do a nationwide quality control based on these registrations in the future as well as to continue to do national trials and answer new questions regarding optimal radiotherapy in early breast cancer.
Along with national screening programmes more and more patients will be eligible for lumpectomy and thereby the need for post-operative radiotherapy will increase. This is a big challenge at a time when the resources are limited. Therefore the accumulating evidence about the use of a moderate hypofractionation schedule after lumpectomy is of great interest Citation[79], Citation[80], but due to the previous serious complications seen in patients treated in the 77 protocols the DBCG radiotherapy group has decided to be cautious before accepting such treatment as a standard. Thus the plan is to evaluate the hypofractionation schedule in a randomized setting with morbidity as the primary endpoint. The suggestion is to compare 40 Gy in 15 fractions in 3 weeks with 50 Gy in 25 fractions in 5 weeks in patients with node negative disease where only irradiation to residual breast is indicated. The morbidity evaluation will include cosmetic outcome, acute and late radiation effect in relation to volume irradiated, quality of treatment technique, interaction with other treatments and patient characteristics as well as identification of genetic profiles – in other words include all the knowledge we have gained from the past.
Another pertinent issue is partial irradiation after lumpectomy in selected low-risk patients. This is a new treatment approach, which is currently being studied in several randomized trials throughout the world Citation[9], Citation[81].Similarly to the hypofractionation protocol described above, the DBCG radiotherapy group also plan a randomized study regarding partial versus whole breast irradiation, again with morbidity evaluation as the primary endpoint. The preparations for this protocol has been underway for a longer time in collaboration with the DBCG surgery group to obtain sufficient knowledge about which method to choose and to find which patients would be eligible Citation[9], Citation[81].
Concluding remarks
During the 30 years time period of DBCG postoperative radiotherapy in breast cancer has moved from orthovoltage treatment based on direct clinical “set up” to megavoltage radiation with 2D and 3D treatment planning Citation[1], Citation[78]. The close collaboration between physicists, radiobiologists and oncologists in the DBCG radiotherapy group has made it possible to design a treatment technique which was tested in the DBCG 82b and c trials and showed to be very effective with respect to tumour control and without major acute and late radiation morbidity. Through these studies, DBCG has contributed with important evidence about the role of postoperative radiotherapy in early breast cancer, and thereby also supported the current paradigm that optimal loco-regional tumour control has influence on survival Citation[82].
The DBCG structure with its scientific radiotherapy committee has given the basis for detailed studies of e.g., detailed evaluation of radiation morbidity in one or two centres in combination with national surveys and register based studies in a population, where the indications and guidelines for radiotherapy have been uniform. This has resulted in a large number of publications regarding clinical, radiobiological and physical aspects in radiotherapy of breast cancer as referred to in this paper. The structure of the radiotherapy group has also created the basis for several doctoral theses dealing with the scientific relevant issues related to radiotherapy in breast cancer Citation[83–89].
The challenge for the DBCG radiotherapy group in the future will especially be the fast growing development in the technical possibilities in radiotherapy. This might help to design more focussed treatment planning in the individual patient with optimized tumour control and reduced radiation dose to critical tissues. In addition continuing work has to be done to tailor the radiation treatment to the extent of surgery and type of systemic therapy guided by the developed prognostic and predictive knowledge.
References
- Kaae S, Johansen H. Breast cancer; five year results: Two random series of simple mastectomy with postoperative irradiation versus extended radical mastectomy. Am J Roentgenol Radium Ther Nucl Med 1962; 87: 82–8
- Cuzick J, Stewart H, Peto R, Fisher B, Kaae S, Johansen H, et al. Overview of randomized trials comparing radical mastectomy without radiotherapy against simple mastectomy with radiotherapy in breast cancer. Cancer Treat Rep 1987; 71: 7–14
- Overgaard, K. [Result of breast cancer treatment at a central hospital.]. Ugeskr Læger. 1961;123:877–9. ( in Danish)
- Johansen H, Kaae S, Jensen M-B, Mouridsen HT. Extended radical mastectomy versus simple mastectomy followed by radiotherapy in primary breast cancer. A fifty-year follow-up to the Copenhagen Breast Cancer randomised study. Acta Oncol 2008; 47: 633–38
- International Commission on Radiation Units and Measurements. ICRU Report 29: Dose specification for reporting external beam therapy with photons and electrons. Bethesda, MD: International Commission on Radiation Units and Measurements; 1978.
- Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. N Engl J Med 1995; 332: 901–6
- Fisher B, Jeong JH, Anderson S, Wolmark N. Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: Updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst 2004; 96: 1823–31
- Blichert-Toft M, Christiansen P, Mouridsen HT. Danish Breast Cancer Cooperative Group – DBCG: History, organisation, and status of scientific achievements at 30-year anniversary. Acta Oncol 2008; 47: 491–96
- Møller S, Jensen M-B, Ejlertsen B, Bjerre KG, Larsen M, Hansen HB, et al. The clinical database and the treatment guidelines programmes of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008; 47: 506–24
- Dombernowsky P, Brincker H, Hansen M, Mouridsen HT, Overgaard M, Panduro J, et al. Adjuvant therapy of premenopausal and menopausal high-risk breast cancer patients. Present status of the Danish Breast Cancer Cooperative Group Trials 77-B and 82-B. Acta Oncol 1988; 27: 691–7
- Mouridsen HT, Rose C, Overgaard M, Dombernowsky P, Panduro J, Thorpe S, et al. Adjuvant treatment of postmenopausal patients with high risk primary breast cancer. Results from the Danish adjuvant trials DBCG 77 C and DBCG 82 C. Acta Oncol 1988; 27: 699–705
- Overgaard M, Christensen JJ, Johansen H, Nybo-Rasmussen A, Brincker H, van der Kooy P, et al. Postmastectomy irradiation in high-risk breast cancer patients. Present status of the Danish Breast Cancer Cooperative Group trials. Acta Oncol 1988; 27: 707–14
- von der Maase, H, Sveinsson, T. [Combined irradiation and chemotherapy as postoperative treatment of breast cancer]. Ugeskr Laeger 1978;140:1676–8. (in Danish)
- McWhirter R. The value of simple mastectomy and radiotherapy in the treatment of cancer of the breast. Brit J Radiol 1948; 21: 599–610
- Ellis F. Dose, time and fractionation: A clinical hypothesis. Clin Radiol 1969; 20: 1–7
- Kirk J, Gray WM, Watson ER. Cumulative radiation effect. I. Fractionated treatment regimes. Clin Radiol 1971; 22: 145–55
- Overgaard M. The clinical implications of non-standard fractionation. Int J Radiat Oncol Biol Phys 1985; 11: 1225–7
- Overgaard M, Bentzen SM, Christensen JJ, Madsen EH. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with postmastectomy irradiation. Radiother Oncol 1987; 9: 1–11
- Overgaard M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose-response relationships on late bone damage. Acta Oncol 1988; 27: 117–22
- Bentzen SM, Thames HD, Overgaard M. Latent-time estimation for late cutaneous and subcutaneous radiation reactions in a single-follow-up clinical study. Radiother Oncol 1989; 15: 267–74
- Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: Impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys 1989; 17: 531–7
- Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J, Nielsen OG, Madsen EH. Quantitative assessment of radiation-induced lung changes by computerized optical densitometry of routine chest x-rays. Int J Radiat Oncol Biol Phys 1996; 34: 421–7
- Bentzen SM, Christensen JJ, Overgaard J, Overgaard M. Some methodological problems in estimating radiobiological parameters from clinical data. Alpha/beta ratios and electron RBE for cutaneous reactions in patients treated with postmastectomy radiotherapy. Acta Oncol 1988; 27: 105–16
- Thames HD, Bentzen SM, Turesson I, Overgaard M, van den Bogaert W. Fractionation parameters for human tissues and tumors. Int J Radiat Biol 1989; 56: 701–10
- Bentzen SM, Overgaard M, Thames HD, Christensen JJ, Overgaard J. Early and late normal-tissue injury after postmastectomy radiotherapy alone or combined with chemotherapy. Int J Radiat Biol 1989; 56: 711–5
- Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-dose factors in radiotherapy: A review of the human data. Radiother Oncol 1990; 19: 219–35
- Bentzen SM, Overgaard M. Relationship between early and late normal-tissue injury after postmastectomy radiotherapy. Radiother Oncol 1991; 20: 159–65
- Bentzen SM, Overgaard M. Early and late normal tissue injury after postmastectomy radiotherapy. Recent Results Cancer Res 1993; 130: 59–78
- Bentzen SM, Overgaard M, Overgaard J. Clinical correlations between late normal tissue endpoints after radiotherapy: Implications for predictive assays of radiosensitivity. Eur J Cancer 1993; 29A: 1373–6
- Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 1996; 88: 918–22
- Johansen J, Bentzen SM, Overgaard J, Overgaard M. Evidence for a positive correlation between in vitro radiosensitivity of normal human skin ibroblasts and the occurrence of subcutaneous fibrosis after radiotherapy. Int J Radiat Biol 1994; 66: 407–12
- Johansen J, Taagehøj F, Christensen T, Overgaard J, Overgaard M. Quantitative magnetic resonance for assessment of radiation fibrosis after post-mastectomy radiotherapy. Br J Radiol 1994; 67: 1238–42
- Johansen J, Bentzen SM, Overgaard J, Overgaard M. Relationship between the in vitro radiosensitivity of skin fibroblasts and the expression of subcutaneous fibrosis, telangiectasia, and skin erythema after radiotherapy. Radiother Oncol 1996; 40: 101–9
- Johansen J, Streffer C, Fuhrmann C, Bentzen SM, Stausbøl-Grøn B, Overgaard M, et al. Radiosensitivity of normal fibroblasts from breast cancer patients assessed by the micronucleus and colony assays. Int J Radiat Biol 1998; 73: 671–8
- Herskind C, Bentzen SM, Overgaard J, Overgaard M, Bamberg M, Rodemann HP. Differentiation state of skin fibroblast cultures versus risk of subcutaneous fibrosis after radiotherapy. Radiother Oncol 1998; 47: 263–9
- Herskind C, Johansen J, Bentzen SM, Overgaard M, Overgaard J, Bamberg M, et al. Fibroblast differentiation in subcutaneous fibrosis after postmastectomy radiotherapy. Acta Oncol 2000; 39: 383–8
- Andreassen CN. Can risk of radiotherapy-induced normal tissue complications be predicted from genetic profiles?. Acta Oncol 2005; 44: 801–15
- Andreassen CN, Alsner J, Overgaard M, Sørensen FB, Overgaard J. Risk of radiation-induced subcutaneous fibrosis in relation to single nucleotide polymorphisms in TGFB1, SOD2, XRCC1, XRCC3, APEX and ATM-a study based on DNA from formalin fixed paraffin embedded tissue samples. Int J Radiat Biol 2006; 82: 577–86
- Andreassen CN, Overgaard J, Alsner J, Overgaard M, Herskind C, Cesaretti JA, et al. ATM sequence variants and risk of radiation-induced subcutaneous fibrosis after postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2006; 64: 776–83
- Alsner J, Rødningen OK, Overgaard J. Differential gene expression before and after ionizing radiation of subcutaneous fibroblasts identifies breast cancer patients resistant to radiation-induced fibrosis. Radiother Oncol 2007; 83: 261–6
- Rødningen OK, Børresen-Dale AL, Alsner J, Hastie T, Overgaard J. Radiation-induced gene expression in human subcutaneous fibroblasts is predictive of radiation-induced fibrosis. Radiother Oncol 2008; 86: 314–20
- Alsner J, Andreassen CN, Overgaard J. Genetic markers for prediction of normal tissue toxicity after radiotherapy. Semin Radiat Oncol 2008; 18: 126–35
- Fisher B. Laboratory and clinical research in breast cancer–a personal adventure: The David A. Karnofsky memorial lecture. Cancer Res 1980; 40: 3863–74
- Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Ann Surg 1907; 46: 1
- Nielsen HM, Overgaard J, Grau C, Christensen JJ, Overgaard M. Audit of the radiotherapy in the DBCG 82 b&c trials-a validation study of the 1,538 patients randomised to postmastectomy radiotherapy. Radiother Oncol 2005; 76: 285–92
- Højris I, Andersen J, Overgaard M, Overgaard J. Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol 2000; 39: 355–72
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Loco-regional recurrence after mastectomy in high-risk breast cancer – risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol 2006; 79: 147–55
- Overgaard M, Christensen JJ, Johansen H, Nybo-Rasmussen A, Rose C, van der Kooy P, et al. Evaluation of radiotherapy in high-risk breast cancer patients: Report from the Danish Breast Cancer Cooperative Group (DBCG 82) Trial. Int J Radiat Oncol Biol Phys 1990; 19: 1121–4
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: Long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol 2006; 24: 2268–75
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997; 337: 949–55
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353: 1641–8
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 2007; 82: 247–53
- Overgaard M. Overview of randomized trials in high risk breast cancer patients treated with adjuvant systemic therapy with or without postmastectomy irradiation. Semin Radiat Oncol 1999; 9: 292–9
- Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol 2008; 26: 1419–26
- Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Andersen J, et al. Tissue micro arrays compared with whole sections and biochemical analyses. A subgroup analysis of DBCG 82 b&c. Acta Oncol 2008; 47: 591–99
- Kyndi M, Sørensen FB, Knudsen H, Alsner J, Overgaard M, Nielsen HM, et al. Impact of BCL2 and p53 on post-mastectomy radiotherapy response in high-risk breast cancer. A subgroup analysis of DBCG82 b&c. Acta Oncol 2008; 47: 608–17
- Kyndi, M, Sørensen, FB, Knudsen, H, Alsner, J, Overgaard, M, Nielsen, HM, et al. Prognostic and predictive value of CA IX in high-risk breast cancer. A subgroup analysis of DBCG82 b&c. Breast Cancer Res 2008; 10:R24.
- Højris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: Analysis of DBCG 82b and 82c randomised trials. Lancet 1999; 354: 1425–30
- Højris I, Sand NP, Andersen J, Rehling M, Overgaard M. Myocardial perfusion imaging in breast cancer patients treated with or without post-mastectomy radiotherapy. Radiother Oncol 2000; 55: 163–72
- Timothy AR, Overgaard J, Overgaard M, Wang CC. Treatment of early carcinoma of the breast. Lancet 1979; 2: 25–6
- Overgaard, M, Overgaard, J. [Local treatment of cancer of the breast]. Ugeskr Læger 1981;143:1401–4. (in Danish)
- Blichert-Toft M, Brincker H, Andersen JA, Andersen KW, Axelsson CK, Mouridsen HT, et al. A Danish randomized trial comparing breast-preserving therapy with mastectomy in mammary carcinoma. Preliminary results. Acta Oncol 1988; 27: 671–7
- Blichert-Toft, M, Rose, C, Andersen, JA, Overgaard, M, Axelsson, CK, Andersen, KW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr 1992;19–25.
- Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol 2001; 19: 1688–97
- Blichert-Toft M, Nielsen M, Düring M, Møller S, Rank F, Overgaard M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008; 47: 672–81
- Johansen J, Overgaard J, Rose C, Engelholm SA, Gadeberg CC, Kjaer M, et al. Cosmetic outcome and breast morbidity in breast-conserving treatment – results from the Danish DBCG-82TM national randomized trial in breast cancer. Acta Oncol 2002; 41: 369–80
- Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol 2000; 39: 349–54
- Johansen J, Overgaard J, Overgaard M. Effect of adjuvant systemic treatment on cosmetic outcome and late normal-tissue reactions after breast conservation. Acta Oncol 2007; 46: 525–33
- Cuzick J, Stewart H, Peto R, Fisher B, Kaae S, Johansen H, et al. Overview of randomized trials comparing radical mastectomy without radiotherapy against simple mastectomy with radiotherapy in breast cancer. Cancer Treat Rep 1987; 71: 7–14
- Ewertz M, Kempel MM, Düring M, Jensen M-J, Andersson M, Christiansen P, et al. Breast conserving treatment in Denmark, 1989–1998. A nationwide population-based study of the Danish Breast Cancer Co-operative Group. Acta Oncol 2008; 47: 682–90
- Overgaard M. Radiotherapy as part of a multidisciplinary treatment strategy in early breast cancer. Eur J Cancer 2001; 37(Suppl 7)S33–S43
- Nielsen, HM, Overgaard, M. [Survey of a Cochrane review concerning sequencing of adjuvant chemotherapy and postoperative radiotherapy in breast cancer]. Ugeskr Laeger 2007;169:3101–4.( in Danish).
- Chen, Z, King, W, Pearcey, R, Kerba, M, Mackillop, WJ. The relationship between waiting time for radiotherapy and clinical outcomes: A systematic review of the literature. Radiother Oncol 2008; 87:3–16.
- Nielsen HM, Christensen JJ, Aagaard T, Thingholm J, Overgaard M, Grau C. A simple method to test if the internal mammary lymph nodes are covered by the wide tangent technique in radiotherapy for high-risk breast cancer. Clin Oncol 2003; 15: 17–24
- Shapiro CL, Hardenbergh PH, Gelman R, Blanks D, Hauptman P, Recht A, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol 1998; 16: 3493–501
- Pedersen AN, Korreman S, Nyström H, Specht L. Breathing adapted radiotherapy of breast cancer: Reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol 2004; 72: 53–60
- Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: -1990s. Int J Radiat Oncol Biol Phys ;69 1950s; 2007: 1484–95
- Thomsen MS, Berg M, Nielsen HM, Pedersen AN, Overgaard M. Ewertz M, et al. @ Post-mastectomy radiotherapy in Denmark: From 2D to 3D treatment planning guidelines of he Danish Breast Cancer Cooperative Group. Acta Oncol 2008; 47: 654–61
- The START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol 2008; 9:331–41.
- The START Trialists’ Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008; 371:1098–107.
- Offersen, BV, Overgaard, M, Kroman, K, Overgaard, J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: A systematic review. (submitted).
- Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol 1994; 12: 2229–34
- Bentzen, SM. Quantitative clinical radiobiology. DMSc thesis, Faculty of Health, University of Aarhus. 1994.
- Johansen, J. Relationship between in vitro radiosensitivity of normal human skin fibroblasts and the occurrence of late normal tissue reactions after radiotherapy. Ph.D. thesis, Faculty of Health, University of Aarhus. 1995.
- Jerzy Skoczylas. Clinical studies of the early and late radiation effect in lung tissue. Ph.D. thesis, Faculty of Health, University of Aarhus. 1995.
- Højris, I. Late morbidity following systemic trfeatment with or without postmastectomy irradiation in patients with breast cancer. Ph.D. thesis, Faculty of Health, University of Aarhus. 1999.
- Andreassen, CN. Can risk of radiotherapy-induced normal tissue complications be predicted from genetic profiles?, Ph.D. thesis, Faculty of Health, University of Aarhus. 2006.
- Nielsen, HM. Failure pattern following adjuvant systemic therapy with or without postmastectomy radiotherapy in high-risk breast cancer patients. Ph.D. thesis, Faculty of Health, University of Aarhus. 2006.
- Kyndi, M. Biological markers predictive of response to adjuvant radiotherapy in breast cancer. Ph.D. thesis, Faculty of Health, University of Aarhus. 2008.
