Abstract
Introduction. Rituximab has significantly improved the prognosis for patients with both indolent and aggressive non-Hodgkin's lymphoma. An economic evaluation was carried out to assess the cost-effectiveness in Sweden of rituximab as maintenance therapy for patients with follicular lymphoma in remission after second line therapy. Materials and methods. The incremental cost and effectiveness of rituximab maintenance therapy versus observation were evaluated in a health-state transition model. Primary effect measures were quality-adjusted life-years (QALY) and life-years gained (LYG). Model state transitions were calculated based on progression-free and overall survival data from the EORTC20981 trial. The analysis was made from the perspective of the healthcare provider, including direct medical costs presented in €, 2007 value. Effects and costs were discounted at a 3% annual rate. The stability of the base case results were tested in one-way and probabilistic sensitivity analyses. Results. The evaluation assessed rituximab maintenance therapy to be associated with an incremental cost per QALY gained of €12 600 and an incremental cost per LYG of €11 200. The average discounted life expectancy for patients on rituximab maintenance was 1.0 year longer than for patients on observation (5.96 vs. 4.94 years). Rituximab maintenance was associated with an additional 0.9 QALY, and total costs per patient were €11 500 higher in the treatment arm, compared to observation. Discussion. The results indicate that rituximab maintenance treatment after successful induction therapy for patients with relapsed/refractory follicular lymphoma in Sweden is cost-effective compared to observation.
Non-Hodgkin's lymphoma (NHL), a group of malignancies of the lymphoid system, ranks among the ten most common cancers in Sweden. There were 1 756 new cases of NHL, including CLL, diagnosed in Sweden in 2005 (3.4% of all cancer cases). Approximately 14% of NHL cases in Sweden are follicular lymphomas (FL) Citation[1]. Follicular lymphoma is a cancer of the B-lymphocytes that occurs almost exclusively in adults. The median age of patients diagnosed with FL grade I-II in Sweden is approximately 63 years, 66 years for FL grade III. FL is an indolent disease with a median survival from diagnosis of 6 – 10 years. Exceptionally, patients presenting with FL in early stages may be cured with radiotherapy and/or chemotherapy, yet the overwhelming number of FL cases are incurable with any current treatment. Although treatment with single agent or combination chemotherapy can achieve complete or partial remissions in most patients, the clinical course is characterised by a continuous pattern of relapses requiring repeated treatment.
Until recently, no particular treatment approach clearly prolonged overall survival for patients with follicular lymphoma. However, in the past few years, rituximab (MabThera®, Rituxan®), a chimeric mouse/human anti-CD20 monoclonal antibody, has made a significant contribution to improving the outcome of patients with both aggressive and indolent NHL; the efficacy of rituximab in combination with chemotherapy has been demonstrated by several randomized trials Citation[2–7]. Rituximab is licensed in the EU in combination with CVP chemotherapy for the first-line treatment of FL and in combination with CHOP chemotherapy for the treatment of patients with CD20-positive diffuse large B-cell lymphoma Citation[8]. Moreover, four randomised trials have investigated rituximab maintenance therapy compared to ‘observation’/‘treatment delayed until relapse’ in patients with relapsed/refractory FL in remission: EORTC20981, GLSG-FCM, SAKK, and LYM-5 Citation[5], Citation[9–11]. All the trials showed that there is an overall benefit from rituximab maintenance therapy. Patients in the trials had received different induction therapies: CHOP, R-CHOP, Rituximab 375 mg/m2, FCM, and R-FCM. The EORTC20981 study included 167 relapsed/refractory FL patients in each treatment arm of the maintenance phase; the other three studies included less than 50 patients in each treatment arm. The EORTC20981 trial Citation[5], Citation[12] was thus found to offer the best evidence base for assessment for a cost-effectiveness evaluation. The trial was designed to investigate the benefit of rituximab added to CHOP chemotherapy for the treatment of relapsed/refractory CD20-positive follicular lymphoma as well as to assess the efficacy of rituximab maintenance therapy in prolonging the duration of response and consisted of two randomisations. At the second randomisation, patients in the rituximab maintenance group received 375 mg/m2 of rituximab once every three months until disease progression or relapse, or for a maximum period of 24 months, while patients in the observation group received no active therapy until disease progression. At disease progression, the patients were eligible for any post-protocol treatment deemed appropriate by the treating physician. The observation arm of the clinical study was thus similar to the prevailing treat-on-relapse approach for patients that have responded to induction therapy; which makes clinical data from EORTC20981 appropriate for determining the cost-effectiveness of rituximab maintenance therapy compared to current clinical practice. The aim of this economic evaluation is to assess the cost-effectiveness, in Sweden, of rituximab added in the maintenance settings for patients with relapsed/refractory FL in remission after second-line therapy.
Material and methods
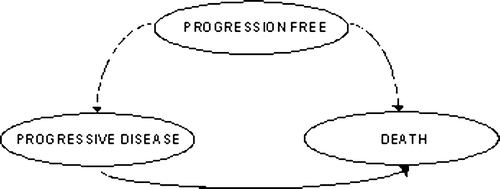
The economic evaluation is based on a previously developed disease model, programmed in Microsoft Excel®, evaluating the incremental cost and effectiveness of rituximab maintenance over observation Citation[13]. The model is a health-state transition model of Markov type with three health states: progression-free (PF), progressive disease (PD) and death. Patients are followed through the health states in monthly cycles over a period of 30 years, in order to capture the full life expectancy of patients. The model structure is presented in .
The hypothetical patient cohort in the economic evaluation is a representation of the population enrolled and randomised in the maintenance phase of EORTC20981, assessed to reflect the patient population likely to be eligible for maintenance therapy in a clinical setting.
The primary endpoints of the economic evaluation are the incremental costs per quality-adjusted life year (QALY) gained and the incremental cost per life year gained (LYG) over the lifetime of the patients. The analysis was made from the perspective of the healthcare provider, i.e. the economic evaluation was based on direct health care costs. Costs are presented in €, 2007 value (€1 = SEK9.25). One-way sensitivity analyses were performed to test the stability of the results and identify the input parameters with particularly influence on the results. A probabilistic sensitivity analysis was also performed to assess the joint stability of the model parameters. Costs and effects were discounted at a 3% annual rate Citation[14].
Clinical data
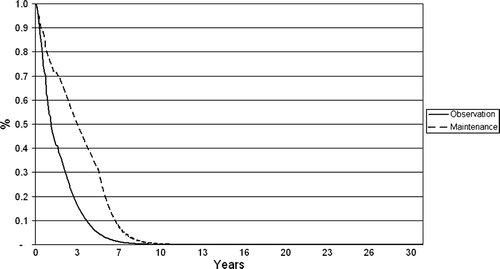
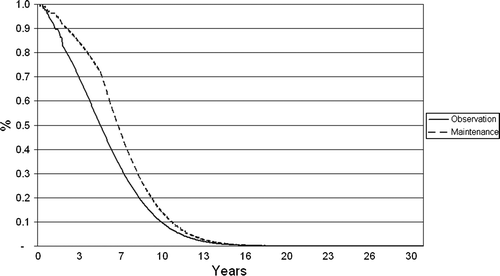
Progression-free survival (PFS) and overall survival (OS) were based on data from EORTC20981. Median PFS from second randomisation in EORTC20981 was 51.5 months in the rituximab arm versus 14.9 months in the observation arm (HR: 0.40, p < 0.001). OS was also significantly longer in the rituximab arm compared to observation: 85% at three years in the rituximab arm versus 77% in the observation arm (HR: 0.52, p = 0.011) Citation[5], Citation[12]; the superior effect was consistent across all subgroups evaluated regardless of risk level at study entry, type of induction regimen, and response level to induction regimen. For the first two years in the model, which was approximately the median duration of follow-up in EORTC20981, Kaplan Meier (KM) data from the trial was used. Thereafter, extrapolation of PFS and OS to the 30-year horizon of the economic model was achieved by fitting a Weibull distribution to the survival data observed during the EORTC20981 trial. The hazards in the model reflect the differences in terms of progression-free and overall survival observed in EORTC20981. and show the results of the extrapolation in the model.
During the maintenance phase, the incidence of grade 3 and 4 adverse events were 37% in the rituximab arm versus 23% in the observation arm while the incidence of serious adverse events were 13% in the rituximab arm versus <1% in the observation arm. The incidence of serious adverse events and the costs they were estimated to incur are presented in .
Cost data
In addition to the cost of the maintenance therapy in itself, direct medical cost consequences of the maintenance therapy include the clinical benefit in terms of reduction in relapses as well as the cost of treating adverse events. Drug costs were Swedish national prices from the National Corporation of Swedish Pharmacies Citation[15], unit costs for out-patient visits and medial procedures were based on prices from the university hospitals of Malmö and Uppsala Citation[16], Citation[17], Inpatient diagnosis-related costs were derived from the national inpatient case-costing database Citation[18] and expert opinion.
Cost for rituximab treatment, including costs for drugs and administration were calculated based on the drug utilisation recorded in the EORTC20981 trial. The average cost per patient on maintenance therapy (drug cost and administration) is presented in .
Table I. Cost of maintenance therapy.
Costs of resource use and hospitalisation for serious adverse events were based on the national inpatient case-costing database Citation[18] and further validated by an expert panel Citation[19]. Costs of adverse event are presented in . The average cost per patient due to serious adverse events was estimated to €587 in the rituximab maintenance arm and €23 in the observation arm. However, due to the longer time of follow-up in patients receiving rituximab and the open label design of EORTC20981, there is a potential bias against rituximab in the estimation of adverse events.
Table II. Cost of serious adverse events during the maintenance phase.
During the maintenance phase, on average 1.6 non-serious adverse events per patient occurred in the rituximab arm and 1.4 events per patient occurred in the observation arm. It was assessed that half of the non-serious adverse events would lead to a medical visit (€225). The total average cost per patient due to non-serious adverse events was estimated to €180 in the rituximab arm and €162 in the observation arm.
Treatment costs upon relapse were included to reflect the cost of therapies likely to be received after disease progression in the trial. Treatment costs upon relapse are based on post-protocol treatments recorded in EORTC20981, and were estimated separately for maintenance and observation, as the type of treatment a patient would receive could depend on whether he/she had received maintenance therapy in second line . The unit costs of treatments received upon relapse were weighted according to the EORTC20981 post-protocol treatment distribution. The average costs per patient per for relapse treatment episode were estimated to €11 249 for the rituximab group and €17 117 for the observation group. It was estimated that the patients would experience a relapses every two years on average, as long as they remained alive, due to the continuous pattern of relapse that is characteristic for follicular lymphoma; the time interval of two years was based on the time to first progression observed in the EORTC20981. The cost of relapse treatment was thus divided into a monthly cost over two year periods, incurred by patients in the progressive disease state of the model.
Table III. Unit costs of treatments received upon relapse Citation[15], Citation[18–20].
Cost of routine management/surveillance for patients in the progression-free state (during maintenance therapy) was assessed to consist of a physician visit (€225) every three months, while it was assumed that patients with progressive disease would visit their physician monthly.
Quality of life data
Utility values (where 1 is considered as one year of perfect health and 0 as dead) were taken from a British study that used the EQ-5D instrument to assess the quality of life in patients with follicular lymphoma Citation[20]. The study presented utility values of 0.805 (n = 132) for progression-free and 0.618 for progressive disease (n = 33). The utility values were directly applied to the PF and PD health states in the model.
Results
The results of the base-case analysis are presented in . The economic evaluation resulted in an incremental cost per QALY gained with rituximab maintenance treatment of €12 600. The incremental cost per LYG was estimated to €11 200. The average discounted life expectancy in the rituximab group was 1.0 year longer than in the observation group (5.96 vs. 4.94 years). Rituximab maintenance was associated with an additional 0.9 QALY compared to the observation group. Total costs per patient were €11 500 higher in the rituximab arm compared to the observation arm. The simulation model hence indicates that maintenance therapy with rituximab for patients in remission after second line treatment of follicular lymphoma in Sweden is cost-effective compared to observation.
Table IV. Base case results
shows the average total discounted cost per patient in the simulation, including treatment of adverse events, treatment costs upon relapse, and routine management. In the rituximab maintenance arm, the total cost per patient amounted to €39 600, while the total cost per patient in the observation group was calculated to €28 200. The cost of maintenance therapy and the treatment costs upon relapse are the greatest cost drivers, while the difference in costs between the two treatment arms of €11 500 was principally due to the cost and administration of maintenance therapy.
Table V. Cost data (€).
The stability of the base-case result was tested in sensitivity analyses (). Decreasing the duration of follow-up and treatment benefit in the model affected the results, yet the incremental cost-effectiveness ratios remained within the acceptable range also under assumptions of a duration of follow-up limited to four years (base-case 30 years) and a duration of treatment benefit of two years (base-case five years), which nevertheless indicates the importance of assessing the long-term clinical consequences of the investigated treatment. Extrapolation of progression-free and overall survival by using a log-logistic distribution lead to a somewhat smaller incremental cost-per-effect measure; the log-logistic regression model resulted in longer estimated life-length of patients than the Weibull regression model and the survival advantage of maintenance therapy was thus extended. Results were robust to 50% changes in cost parameters.
Table VI. One-way sensitivity analysis.
A probabilistic sensitivity analysis was performed to test the stability of the deterministic (base-case) results by assessing the joint uncertainty of the underlying clinical, quality-of-life and cost parameters. The covariate parameters in the Weibull regression model were varied according to an inverse normal distribution. The clinical data on adverse events and post protocol treatments were drawn from beta distributions, and quality-of-life data were varied following a trimmed normal distribution; with parameter variation based on the original data from the clinical trial and the quality-of-life study. Cost data were varied within an estimated range according to triangular or linear distributions.
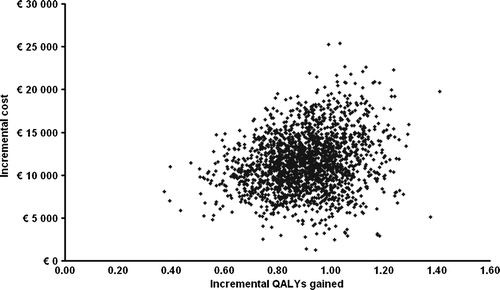
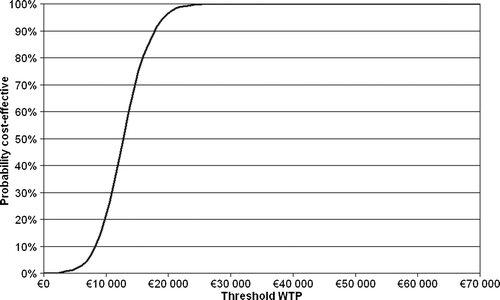
The probabilistic sensitivity analyses showed that rituximab maintenance therapy was more effective with an incremental effectiveness of more than 0.37 QALY in 100% of the performed simulations. The incremental cost of rituximab maintenance treatment did not exceed €25 400 in any case (). The average incremental effect in the probabilistic analysis was 0.9 QALY and the average incremental cost was €11 500.
The acceptability curve derived from the probabilistic sensitivity analysis () demonstrates that rituximab maintenance treatment would incur a cost per QALY gained of less than €25 400 in 100% of the simulated cases. The willingness-to-pay (WTP) threshold for which a treatment is considered cost effective is generally considered to be approximately €54 000 (SEK 500 000) in Sweden Citation[21]. This indicates that the calculated base-case cost-effectiveness results are stable and that rituximab maintenance therapy can be considered a cost-effective treatment for patients with FL in remission after second line therapy.
Discussion
The cost-effectiveness of rituximab maintenance therapy in patients with relapsed/refractory follicular lymphoma who have responded to induction therapy has previously not been assessed in a Swedish setting. The results of the economic modelling indicate that rituximab maintenance therapy is a cost effective intervention, with an incremental cost per QALY gained over observation of €12 600. For comparison, an Italian study from 2007, likewise based on the EORTC20981 trial and similar model design, assessed the cost effectiveness of rituximab maintenance to €11 100 per QALY gained Citation[13].
The aim of a health economic model is to model clinical practice and clinical effects as accurately as possible, nevertheless, a model is a simplification of reality and although the most valid clinical data available is in many cases data from clinical trials, these data may not always be completely comparable to clinical practice. For example, the assessment of treatment received upon relapse, based on data from the EORTC20981 trial, might not be completely equivalent to what would be given to Swedish patients today since clinical practice might have changed since the trial was conducted. However, due to the small difference in average costs of treatment received upon relapse between the two treatment arms in the study, it is unlikely that variations in treatment upon relapse would greatly affect the cost difference between treatment arms. Likewise, the costs of adverse events in the economic model were based on clinical expert estimates and data from the Swedish inpatient case-costing database and not empirically verified, but as shown in the sensitivity analysis, variations in costs of adverse events had limited impact on the results of the analysis.
The economic evaluation does not include indirect costs. In Sweden approximately 55% of patients are under 65 years of age at diagnosis of FL. Thus, including cost of production loss would have had a certain influence on the model results. Since all patients experienced relapses the difference between the treatment arms would have been limited, but assumable in favour of ritixumab since patients treated with rituximab had a prolonged time to relapse/refraction. The greatest indirect cost is generally the cost of mortality and since survival was prolonged for patients treated with rituximab in the present simulation, it can be considered a conservative approximation not to include indirect costs in the analysis. The significant clinical benefit for rituximab maintenance therapy demonstrated in the EORTC20981 trial reassures the stability of the cost-effectiveness results.
Acknowledgements
Declaration of interest: The study was financed by Roche AB, Sweden
References
- Svenska Lymfomregistret Rapport för år 2000-2005 (the Swedish lymphoma registry report 2000-2005), Svenska Lymfomgruppen, Sveriges Onkologiska Centra [Online]. Available from: http://www.ocsyd.lu.se/VP-verksamhet/Kvalitetsreg/RapportLymfom2000-2005.pdf.
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006; 7: 379–91
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006; 24: 3121–7
- Intragumtornchai T, Bunworasate U, Nakorn TN, Rojnuckarin P. Rituximab-CHOP-ESHAP vs CHOP-ESHAP-high-dose therapy vs conventional CHOP chemotherapy in high-intermediate and high-risk aggressive non-Hodgkin's lymphoma. Leuk Lymphoma 2006; 47: 1306–14
- van Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 intergroup trial. Blood 2006; 108: 3295–301
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005; 106: 3725–32
- Fisher RI. Overview of Southwest Oncology Group Clinical Trials in non-Hodgkin Lymphoma. S0016. A phase III trial of CHOP vs CHOP + rituximab vs CHOP + iodine131-labeled monoclonal anti-B1 antibody (tositumomab) for treatment of newly diagnosed follicular NHL. Clin Adv Hematol Oncol 2005; 3: 544–6
- Committee for medicinal products for human use post-authorisation summary of positive opinion for Mabthera [Online]. [cited 2007 April]; Available from: www.emea.europa.eu/pdfs/human/opinion/20477106en.pdf.
- Forstpointner R, Unterhalt M, Dreyling M, Bock HP, Repp R, Wandt H, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006; 108: 4003–8
- Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004; 103: 4416–23
- Hainsworth JD, Litchy S, Shaffer DW, Lackey VL, Grimaldi M, Greco FA. Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin's lymphoma–a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2005; 23: 1088–95
- EORTC20981: Clinical Study Report – EORTC20981 (M39022) – Chimeric anti-CD20 monoclonal antibody (MabThera) in remission induction and maintenance treatment of relapsed follicular non-Hodgkin's lymphoma: A phase III randomized clinical trial – Intergroup Collaborative Study. Research report 1016350 – December, 2005.
- Berto P, Lopatriello S, Arcaini L, Del Poeta G, Martellin M, Gargantini L, et al. Costo-efficacia di rituximab nella terapia di mantenimento in soggetti affetti da linfoma non-Hodgkin follicolare refrattario o recidivante. PharmacoEconomics-Italian Research Articles 2007; 9: 9–19
- General guidelines for economic evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2) [Online]. [cited 2007 April]; Available from: http://www.lfn.se/upload/Foretag/ENG_lfnar2003-eng.pdf.
- Läkemedelsportalen (Swedish national pharmaceuticals price list) [Online]. [cited 2007 April]; Available from: www.fass.se.
- Prislista Akademiska sjukhuset i Uppsala (Uppsala University Hospital price list) [Online]. [cited 2007 April]; Available from: www.externt9.lul.se/svn/uppsala07.xls.
- Prislista Universitetsjukhuset MAS-Malmö (Malmö University Hospital price list) [Online]. [cited 2007 April]; Available from: www.srvn.org/prislista.htm.
- KPP-databasen (the national inpatient case-costing database), The Swedish Association of Local Authorities and Regions [Online]. [cited 2007 April]; Available from: stat.skl.se/kppprod/index.htm.
- Martin Erlanson, Norrlands University Hospital, Umeå; Hans Hagberg, Uppsala University Hospital; Uppsala; Eva Kimby, Karolinska University Hospital, Huddinge; Thomas Relander, Lund University Hospital, Lund; [personal communication, April 2007].
- Utility values in Follicular Lymphoma. Oxford Outcomes Ltd 2005.
- U Persson, J. Hjelmgren [Health services need knowledge of how the public values health]. Lakartidningen 2003;100:3436–7.