Abstract
Purpose. To investigate the impact of initial tumour characteristics and loco-regional radiotherapy on long-term survival following breast cancer diagnosis. Methods and materials. This study was conducted among 6 800 French women from a cohort of 7 711 subjects diagnosed at the IGR with breast cancer between 1954 and 1983 and followed-up until January 2004. Overall mortality in the cohort was compared with that in the French general population using Standardized Mortality Ratios (SMR) and the Absolute Excess Risk (AER) estimated by Poisson regression. Results. During the 1954–2004 follow-up period, 5 436 women died. Mortality was 3.15-fold higher in the cohort than in the general female population in France. It decreased from 6.86 to 1.26 during the first 30 years of follow-up then rose again to 1.60. Both SMRs and AERs were more than 2-fold higher in women who had received radiotherapy during initial treatment than in those who had not, this difference being higher for women treated before 1976 than afterwards (p < 0.0001). They (SMRs and AERs) were also higher for subjects who had stage II, III or IV lesions than for those with less advanced tumours. Conclusion. The results of this study suggest that the excess deaths observed during the first two decades are closely linked to the initial clinical characteristics of the tumour and to radiotherapy. The late increase in mortality may be partially due to deleterious late effects of radiotherapy.
Breast cancer is the most frequent cancer among women in developed countries accounting for about 30% of all malignant diseases in French women Citation[1]. Survival after breast cancer has improved over the past few decades. This improvement is assumed to be the result of both earlier diagnosis and treatment effects, particularly the use of adjuvant chemo- and hormonotherapy. Although radiotherapy is known to reduce 3-fold the risk of loco-regional recurrence, its real impact on long-term survival has been widely debated Citation[2–5]. A worldwide overview of randomised trials on early breast cancer treatments recently showed that radiotherapy increased long-term survival mainly in patients at a high risk for loco-regional recurrence Citation[6]. This meta-analysis also showed an increased risk of late heart disease and second cancers, as previously reported in other studies Citation[3], Citation[7–10].
Excess mortality after breast cancer treatment, as compared with that observed in the general population, decreases during the first two decades after disease onset Citation[11–18]. Some authors claim that long-term mortality could attain that observed in the general population, suggesting that patients could then be considered cured Citation[14], Citation[19]. However, breast cancer loco-regional recurrences or metastases can arise decades after the initial treatment and some authors reported on excess mortality from breast cancer up to 40 years later among women younger than 40 at diagnosis Citation[20]. The long-term curability of breast cancer therefore remains controversial.
In a previous report on a single hospital-based cohort of patients diagnosed between 1954 and 1967 Citation[18], we showed that excess mortality decreased during the first two decades following treatment and then subsequently increased. This cohort has been extended to include a greater number of women treated between 1968 and 1983. Medical follow-up was updated in 2004. The aim of the present study was to investigate the impact of initial tumour characteristics and of loco-regional radiotherapy on long-term survival following breast cancer diagnosis.
Patients and methods
Patients
Between 1954 and 1983, the treatment protocol at the Institut Gustave Roussy (IGR) was based on the TNM (UICC) classification and on the histological lymph node status. Patients were divided into three groups. The first group included patients with T0, T1, T2, T3 lesions, with a clinical tumour size measuring less than 7 cm, and clinically classified as N0, N1, and M0. These women had undergone surgery (including axillary lymph node dissection plus either a mastectomy or lumpectomy always followed by breast radiotherapy). After surgery, loco-regional radiotherapy, including chest wall/breast and axillary, supraclavicular and internal mammary chain lymph nodes, had been delivered exclusively to patients with histologically positive axillary lymph nodes. The second group included patients with a clinical tumour size exceeding 7 cm or tumour with total adherence to the pectoral muscle or skin involvement, but no distant metastases. These patients had received preoperative radiotherapy followed by a mastectomy and axillary lymph node dissection. The third group included all other patients with inflammatory breast cancer and M1 disease. These patients had generally been treated with radiotherapy alone, combined with chemotherapy and hormonotherapy, but some patients with metastases had not received loco-regional irradiation.
Until 1969, the surgical procedure was Halsted's operation and patients with a medial or internal tumour with histologically involved axillary lymph nodes had undergone dissection of the internal mammary chain. After 1969, a Patey mastectomy was performed which includes axillary node dissection. A wide tumourectomy with axillary lymph node dissection was systematically performed in patients with a tumour measuring less than 25 mm, after a randomised trial comparing mastectomy and conservative treatment (1972–1978) Citation[21]. Radiotherapy delivered to the breast was indicated after breast-conserving surgery. Lymph node irradiation depended on the axillary lymph node status, as mentioned above. A dose of 45 to 50 Gy over 4–5 weeks was delivered. For breast conservative treatment, an additional boost dose of 15 Gy was usually delivered to the tumour bed. In premenopausal women with histologically positive lymph nodes, ovarian ablation was performed using pelvic radiotherapy or surgery.
From 1954 to 1957, radiotherapy was delivered with a 200 kV x-ray machine (orthovoltage). From 1958 onwards, Cobalt-60 units were used. Since 1977, the internal mammary chain has been treated with a direct field with a mixed beam of Co-60 photons and electrons of the appropriate energy.
Chemotherapy was not used systematically as adjuvant therapy (less than 1% before 1977 and around 20% per year subsequently). Tamoxifen was introduced around 1975, mainly for postmenopausal patients with locally advanced or metastatic breast cancer.
A population of 7 711 consecutive breast cancer patients initially treated at the IGR between 1954 and 1983 was prospectively followed-up through medical records Citation[22]. We excluded 911 women born in a foreign country and not living in France at the time of the breast cancer treatment, because of very frequent loss to follow-up. All the 6 800 remaining women were included in this analysis. Eighty-five percent of them had been regularly seen at our long-term follow-up clinics at the IGR, where they were screened yearly during the first five years, then every two or three years thereafter. Their follow-up data were complete in the medical records. To collect follow-up data, we first abstracted information directly from the medical records where demographical characteristics, clinical and pathological features of the breast cancer, initial treatment and the health status were recorded. The vital status of patients on January 1, 2004 was also obtained from the medical records. Special efforts were then expended to establish the recent medical status of all patients who were lost to follow-up. Patients who were still lost to follow-up after consulting their physicians, were traced by verifying the municipal registries which fully cover the French population. These registries supplied information about the vital status and the most recent address of the patient. Next, the patients who were still alive were invited to visit the IGR, where information on their medical status could be completed. For 998 women who were not regularly seen at our follow-up clinic, data in the medical records were incomplete. These 998 women (15%) were considered lost to follow-up before January 1, 2004. Patients’ identification data were used to update vital status for all breast cancer patients from the National Institute of Statistics and Economic Studies (INSEE). This process enabled us to obtain vital status for all breast cancer patients on January 1, 2004 including 905 of the 998 with incomplete medical follow-up.
Methods
Overall survival rates were estimated with the Kaplan-Meier method Citation[23]. Mortality in our cohort was compared to overall mortality in the French general population using Standardized Mortality Ratios (SMRs) and Excess Absolute Risks (EARs) of death estimated by Poisson regression Citation[24]. The reference was the French mortality rates according to age categories and 5-year periods obtained from the French Institute of Health and Medical Research (INSERM). Each woman's contribution to person-years at risk (PYR) began from the date of the start of breast cancer treatment and ended when the first of the following events occurred: January 1, 2004, or the date of death, or the date of last medical examination (loss to follow-up) or the 94th birthday. This was done, because national age-specific death rates are not accurately known for people aged 94 years or more.
Taking into account PYR of observation in the cohort (by age and calendar period), the expected number of deaths were computed using age and calendar period-specific mortality rates. The SMR was defined as the ratio between observed and expected deaths. The EAR was defined as the difference between the observed and expected deaths divided by the number of PYR and was expressed in percentage. The confidence intervals (CI) of the SMRs and EARs were derived using the exact maximum of likelihood method Citation[25]. Regression analysis of variation in SMRs was performed assuming that the observed number of deaths followed Poisson distribution. All tests for heterogeneity (PHET) and trends (PTrend) were two sided.
Data analysis was performed using SAS ® software procedures for internal comparisons and the EPICURE® statistical software package for external comparisons Citation[26].
Results
The main characteristics of the cohort are shown in . The average age at diagnosis was 56 years (range 21–94). Thirty-seven percent of the women were under 50 years of age at diagnosis. The diagnosis was stage I, stage II and stages III and IV disease respectively in 11, 51, and 38% of patients. During the period from 1954 to 1957, 50% of the women had stage III or IV disease, whereas this figure dropped to only 24% after 1977. Stage I disease accounted for respectively 2, 5 and 21% for the three consecutive periods of diagnosis (p for trend < 0.0001). Nine percent of the women had distant metastases at diagnosis. Among the entire population, 51% had left-sided and 2% synchronous bilateral breast cancer. Seventy-six percent of women had been treated surgically and 72% had received radiotherapy. Among the latter women, 8% had been treated with orthovoltage. Radiotherapy was delivered to 68, 62 and 86% of cases respectively with stage I, II and III-IV disease (PTrend<0.0001). Radiotherapy was delivered systematically after breast-conserving surgery. Sixty-one percent of women had received radiotherapy before or after radical mastectomy, and 86% of those who had never undergone surgery (stages III and IV). Radiotherapy was more frequently delivered to patients treated between 1954 and 1956 (79%) compared to 70% during the period after 1956 (PHET<0.0001).
Table I. Initial characteristics of 6 800 breast cancer patients.
On January 1, 2004, less than 2% of the women (93) were definitively lost to follow-up and 5 436 (80%) had died. For the entire cohort, the median follow-up estimated by the inverted Kaplan-Meier method was 10 years (1–48 years). The total number of person-years at risk was 82 039. The 10-year, 20-year, 25-year and 30-year survival rates were respectively 46% (95%CI: 45–47%), 31% (95%CI: 30–32%), 25% (95%CI: 24–27%) and 20% (95%CI: 19–21%). Among the 518 women still alive 30 years after breast cancer treatment, 97% had stage I or II disease, 86% a tumour measuring less than 5 cm, 97% a maximum of one involved lymph node and all of them were free of distant metastases at diagnosis. Among the 30-year survivors, 199 had a complete medical follow-up for at least 25 years after breast cancer treatment. Among them, only 26 (13%) developed a local recurrence or distant metastasis, and 34 (17%) a contralateral breast cancer.
During the entire follow-up period, 701 women developed a contralateral breast cancer, and it occurred in more than 80% of them during the first decade of follow-up. Women who developed a contralateral breast cancer were significantly younger at diagnosis than the others (PHET<0.0001) and had more advanced disease (45% stage III and IV versus 39% for the others), but the frequency of contralateral lesions did not vary substantially according to the period of initial treatment of the breast cancer.
Excess mortality for the total period of follow-up
Mortality in the entire cohort was 3.15-fold higher (95% CI [3.07–3.24]) than that of the general female population in France (); the EAR was 4.6 deaths per 100 PYR (95%CI: 4.4–4.8). SMRs decreased significantly for an older age at diagnosis, whereas the EARs were significantly higher for older women at diagnosis. Women treated before 1957 had higher SMRs and EARs than the others. Both SMRs and EARs were higher for stage III and IV than for less advanced tumours.
Table II. Standardized mortality rate (SMR) among 6 800 women according to clinical parameters and radiotherapy.
SMRs and EARs were similar for patients who had right-sided or left-sided tumours, whereas they were statistically significantly higher in patients who had a tumour located in the external quadrants (3.3 and 4.8%) than in those with tumours located into the medial or internal quadrants (2.7 and 3.9%). Both SMRs and EARs were more than 2-fold higher for women who had received radiotherapy than for those who had not. This difference was higher for women treated before 1976 than later (p < 0.0001) and for those with stage II, III or IV disease. No significant interactions were observed between radiotherapy and the other parameters (data not shown).
Variations in excess mortality according to follow-up
For the entire cohort, the excess deaths observed decreased significantly with time since the diagnosis of breast cancer (), without ever reaching the mortality of the general female population in France, and rose again after 35 years of follow-up. The EARs also decreased significantly (p < 0.0001) with time since the diagnosis and then increased after 35 years of follow-up. The EAR was 2.8% after 35 years of follow-up. The SMR decreased significantly faster among younger than older women but between 21 and 25 years after treatment it was still 3.0 among women of 40 years or younger at diagnosis (). Based on few cases, we observed an increase in SMRs after 35 years of follow-up ().
Figure 1. Ratio of observed to expected deaths (left panel) and Excess absolute risk (right panel) between 0 and 40 years after a primary breast cancer (n = 6800).
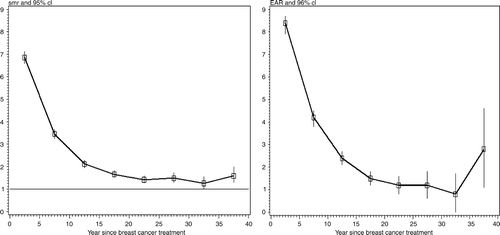
Table III. Total number of observed and expected deaths, SMR and EAR according to age at diagnosis and follow-up (N = 6 800).
During the first 5 years of follow-up, the SMR was significantly higher for women treated between 1954 and 1956 (), than for those treated between 1957 and 1976, and later. Up to 25 years of follow-up, no major differences in risks were observed between the three periods of diagnosis ().
Table IV. Total number of observed and expected deaths SMR and EAR according to follow-up and the period of initial treatment of breast cancer (N = 6 800).
During the first 25 years of follow-up both SMRs and EARs decreased faster in women who had stage III or IV breast cancer than in those who had earlier stages (data not shown).
The side on which the breast cancer occurred did not play a role in the temporal pattern of survival; SMRs in two groups of patients were similar whatever the follow-up period (data not shown). During the first 5-year period of follow-up, the SMR was higher in patients with an external or medial tumour (SMR = 7.2, 95%CI: 6.9–7.5) than in those with an internal tumour (SMR = 5.3, 95% CI: 4.8–5.8), but this difference disappeared subsequently (data not shown).
Among the non-irradiated patients, mortality reached that of the general population after 20 years of follow-up, whereas it remained significantly elevated during the whole follow-up period in irradiated women. The 21–25-year EAR was significantly higher for women who had been treated with radiotherapy than for those who had not (). There was, however, a borderline increase after 35 years in women who had not been treated with radiotherapy.
Table V. Total number of observed and expected deaths SMR and EAR according to follow-up with regard to radiotherapy as part of the initial treatment (N = 6 800).
Multivariate analysis
In a multivariate analysis, a younger age at diagnosis, an earlier calendar period at diagnosis, a more advanced stage breast cancer and radiotherapy were associated with a higher risk of death (), whereas the location of the tumour in the breast ceased to be associated with mortality.
Table VI. Relative risk and adjusted relative risk among 6 800 women according to clinical parameters and radiotherapy.
The same results were obtained when data were stratified on the treatment period and the tumour stage. After the first two decades of follow-up, the risk of death remained higher for women who had received radiotherapy (RR = 1.4; 95%CI: 1.3–1.5).
During the follow-up period, 567 of the 701 women who developed a contralateral breast cancer died. The excess deaths observed were significantly higher among these women (SMR = 3.6; 95% CI: 3.3–3.9) than among the other women (SMR = 3.1; 95%CI: 3.0–3.2).
This difference was observed up to 30 years after breast cancer treatment and particularly for women aged 50 or under: SMR: 9.2 (95% CI: 8.1–10.6) vs. 5.9 (95% CI: 5.6–6.2).
Discussion
In a cohort of 6 800 women treated for breast cancer at the IGR between 1954 and 1983, mortality was 3.15-fold higher than that in the general female population in France. The excess deaths observed decreased significantly with time since the diagnosis but never attained the mortality rate of the general female population of France, even after 30 years of follow-up.
A younger age at diagnosis, an earlier calendar period during treatment, a more advanced stage breast cancer and initial treatment including radiotherapy were associated with a higher SMR.
Like any hospital-based study, our analysis is subject to some biases. Initially, the cohort comprised 7 711 women treated for breast cancer. We excluded from the analysis all women born in foreign countries and not living in France since they were more frequently lost to follow-up. However, these limitations are counterbalanced by the advantages of having a large hospital-based cohort with a very long-term and complete follow-up and standardized treatment protocols. Among the patients included, less than 2% were lost to follow-up, which is low as follow-up exceeded 40 years.
In a previous study Citation[18] on 2 151 women treated at the IGR between 1954 and 1967 and followed-up until 1983, mortality decreased during the first two decades after the diagnosis attaining that of the general population after 20 years of follow-up. However, in this first study, late mortality rose after the 25th year of follow-up.
Other long-term studies
Of the few other studies on long-term mortality after breast cancer treatment, all but two, published in the 70's and 80's, suggested that the excess mortality in women with breast cancer compared to that of the general population decreases during the first two decades Citation[9], Citation[12–17], Citation[27]. In the studies on patients treated before our series, the relative risks were higher than those evidenced in our cohort and late mortality remained higher than that of the general population after a follow-up of at least 20 years () Citation[12], Citation[13].
Table VII. Studies of long-term mortality after breast cancer compared with expected survival for the general population with the same age distribution
In the studies on patients treated virtually during the same period as our series, late mortality attained that of the general population within 10–20 years of follow-up Citation[14], Citation[16]. However, other studies showed that late mortality remained higher than unity with follow-up not exceeding 20 years Citation[17], Citation[28]. In the series of women treated more recently, risks were lower than in our study Citation[9]. For instance, in a recent INSERM expert report Citation[29], excess deaths ranged from 2.4 to 4.9% during the first 12 years after breast cancer treatment among the French women diagnosed during the 1983–1994 period.
Age at breast cancer diagnosis and late mortality
As shown in , the SMR in our study was significantly higher for women younger than 40 at diagnosis (SMR = 12.4, 95%CI: 11.4–13.6), but the EAR was significantly higher for older women (5.5%; 95%CI: 5.3–5.8) due to the small number of expected deaths among the youngest women. For these younger women, the excess deaths observed decreased significantly with time since the diagnosis and rose again after 35 years of follow-up, as shown in (SMR = 2.0: 95% CI: 1.1–3.4). The higher number of excess deaths observed among younger women could be partially due to the incidence of contralateral breast cancer which affected younger women more frequently. In a cohort study conducted on the National Finnish Cancer Registry and restricted to 18 578 women treated for a breast cancer before the age of 50, Brenner et al. showed that mortality remained higher than expected throughout at least 40 years after the diagnosis Citation[27]. This result is consistent with the widely held belief that breast cancer is a more aggressive disease in younger women than in older women. In a recent study on more than 400 000 women registered in the SEER program for breast cancer, Schairer et al. reported that the proportion of deaths due to breast cancer decreased with age at diagnosis Citation[30]. Langlands et al. did not directly study the effect of age but provided SMRs and EARs according to the menopausal status at the diagnosis of breast cancer. The pattern of risks they found was similar to ours: an increasing SMR for premenopausal women compared to postmenopausal women Citation[17]. Circulating female hormones may play a role in the aggressiveness of breast tumours. Among 57 068 women from the nationwide Swedish cancer registry with a breast cancer diagnosed between 1960 and 1978, Adami et al. found that relative survival in women aged 45 to 49 years exceeded that of the younger women and suggested that hormonal depletion at this age might slow down the growth rate of hormone-dependent tumours Citation[31]. However, they also showed that relative survival rates were lower in older women. It has previously been shown, based on the same registry of women treated between 1959 and 1963, that younger women had higher relative survival rates Citation[32]. In agreement with this finding, Rutqvist et al. in their study based on the Norway cancer registry found that the proportion of cured women was higher among younger women Citation[28].
Other factors and late mortality
In our series, mortality was significantly related to the period of treatment, the disease stage at diagnosis and radiotherapy. These three parameters are closely related. As shown in our study, breast cancer was diagnosed at an earlier stage in more recent periods. In addition, surgery and radiotherapy techniques have improved over time and their indications varied according to the stage of the disease. In the multivariate analysis, and as expected, mortality remained significantly related to the stage of the disease after taking into account the period of diagnosis, the initial age and radiotherapy.
Significant differences in mortality appear, particularly during the first 5 years of follow-up between the different stages, with greater excess mortality for stages III and IV. This difference in mortality does not persist after the first decade of follow-up. Our results are consistent with the findings of Langlands et al. Citation[17].
Radiotherapy effect
In our study, mortality was significantly related to radiotherapy. The relative risk was 1.7 95%CI: [1.6–1.8] for the entire period of follow-up and after 20 years of follow-up, the risk of death remained 1.4-fold higher among women previously treated with radiotherapy. Darby et al. demonstrated that the late increase in mortality among women treated during the past decades is mostly due to the late effects of old radiotherapy modalities and they are observed at least 10 years after treatment through an adverse effect on the heart and lungs Citation[7]. However, our study analysed mortality whatever the cause. More detailed information will be obtained when the specific cause of death is analysed. In our series, a part of the difference between irradiated and non-irradiated women might be due to initially advanced disease which has only been partially taken into account in the multivariate analysis conducted in a non-randomised population.
Can long-term survivors be considered cured of their breast cancer in this long-term follow-up study? Mortality in these women never reached that of the general female population in France. However, after 30 years of follow-up, 518 women from the cohort are still alive, and 199 of them had a complete medical follow-up. These women had less extensive tumours than the others, none had distant metastases at diagnosis, and only 26 (13%) developed a local recurrence or a contralateral breast cancer. A limitation in our study is that the curability of breast cancer could not be truly estimated as we did not analyse the long-term effects of more recent treatments, such as chemo- or hormonotherapy. However, these treatments were used for the most recent relapses. This might explain the decrease in the number of deaths during the last years of follow-up, which could be related to delayed death associated with effective treatments.
In conclusion, the total excess deaths observed among women treated for breast cancer decreased over time without reaching that of the general population and seems to rise again after 35 years of follow-up. Our data showed that the excess deaths observed during the first two decades are closely linked to the initial clinical characteristics of the tumour at presentation. The late increase in mortality might partially be due to deleterious late effects of radiotherapy, as practiced during the period analysed. The enhanced risk among long-term survivors highlights the importance of routinely following breast cancer patients throughout their lifetime for early detection of late deleterious effects of breast cancer treatments.
Acknowledgements
Houda Boukheris receiced a fellowship from the International Agency for Research on Cancer (IARC). We are grateful to Pauline Brindel, Françoise Doyon, Ziyan Famy, Claudine Gloaguen, Catherine Guibout, Nadia Haddy, Haouari Zineddine, Pascale Jean, Martine Labbé, Catherine Paoletti for their assistance in completing the database and obtaining the follow-up data, and to Lorna Saint Ange for editing.
References
- Remontet L, Esteve J, Bouvier AM, et al. Cancer incidence and mortality in France over the period 1978–2000. Rev Epidemiol Sante Publique 2003; 51: 3–30
- Arriagada R, Le MG, Guinebretiere JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol 2003; 14: 1617–22
- Cuzick J, Stewart H, Peto R, et al. Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep 1987; 71: 15–29
- Early Breast Cancer Trialists’ Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomised trials. Lancet 2000;355(9217):1757–70.
- Rutqvist LE, Rose C, Cavallin-Stahl E. A systematic overview of radiation therapy effects in breast cancer. Acta Oncol 2003; 42: 532–45
- Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366(9503):2087–106.
- Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005; 6: 557–65
- Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005; 97: 419–24
- Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys 2006; 64: 1081–91
- Rubino C, de Vathaire F, Shamsaldin A, Labbe M, Le MG. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer 2003; 89: 840–6
- Adair F, Berg J, Joubert L, Robbins GF. Long-term follow-up of breast cancer patients: The 30-year report. Cancer 1974; 33: 1145–50
- Al Fouadi A, Sorahan T, Prior P. Long-term survival of women diagnosed with breast cancer 1936–50. Lancet 1987; 1(8541)1096–97
- Brinkley D, Haybittle JL. Long-term survival of women with breast cancer. Lancet 1984; 1(8386)1118
- Duncan W, Kerr GR. The curability of breast cancer. Br Med J 1976; 2(6039)781–3
- Ferguson DJ, Meier P, Karrison T, Dawson PJ, Straus FH, Lowenstein FE. Staging of breast cancer and survival rates. An assessment based on 50 years of experience with radical mastectomy. JAMA 1982; 248: 1337–41
- Hibberd AD, Horwood LJ, Wells JE. Long term prognosis of women with breast cancer in New Zealand: Study of survival to 30 years. Br Med J (Clin Res Ed) 1983; 286(6380)1777–9
- Langlands AO, Pocock SJ, Kerr GR, Gore SM. Long-term survival of patients with breast cancer: A study of the curability of the disease. Br Med J 1979; 2(6200)1247–51
- Le MG, Hill C, Rezvani A, Sarrazin D, Contesso G, Lacour J. Long-term survival of women with breast cancer. Lancet 1984; 2(8408)922
- Brinkley D, Haybittle JL. The curability of breast cancer. World J Surg 1977; 1: 287–9
- Rutqvist LE, Wallgren A. Long-term survival of 458 young breast cancer patients. Cancer 1985; 55: 658–65
- Sarrazin D, Le MG, Arriagada R, et al. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol 1989; 14: 177–84
- Rubino C, de Vathaire F, Diallo I, Shamsaldin A, Le MG. Increased risk of second cancers following breast cancer: Role of the initial treatment. Breast Cancer Res Treat 2000; 61: 183–95
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II–The design and analysis of cohort studies. IARC Sci Publ 1987; 82: 1–406
- Moolgavkar SH, Venzon DJ. A method for computing profile likelihood-based confidence bounds. Ann Stat 1987; 15: 346–59
- Preston DL, Lubin JH, Pierce DA, McConney ME. EPICURE. Generalized regression models for epidemiological data. Seattle; Hirosoft International Corporation: 1991.
- Brenner H, Hakulinen T. Are patients diagnosed with breast cancer before age 50 years ever cured?. J Clin Oncol 2004; 22: 432–8
- Rutqvist LE, Wallgren A, Nilsson B. Is breast cancer a curable disease? A study of 14,731 women with breast cancer from the Cancer Registry of Norway. Cancer 1984; 53: 1793–800
- Expertise Collective. Cancers: Pronostics à long terme. ParisFrance: Les éditions Inserm, 2005.
- Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst 2004; 96: 1311–21
- Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med 1986; 315: 559–63
- Adami HO, Malker B, Meirik O, Persson I, Bergkvist L, Stone B. Age as a prognostic factor in breast cancer. Cancer 1985; 56: 898–902
